MOLECULAR MASS SPECTROSCOPY
MOLECULAR Mass Spectroscopy
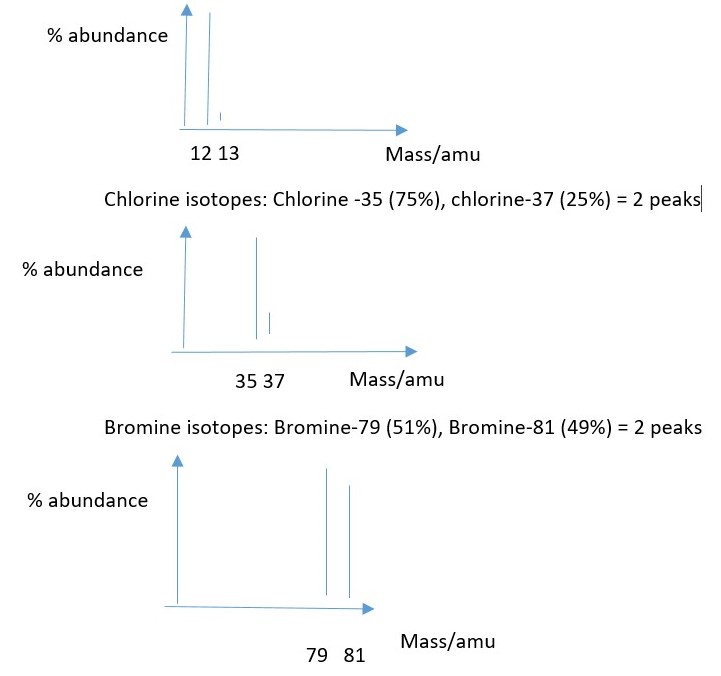
Atomic mass spectroscopy reveals the mass of different isotopes of atoms of an elements and their percent abundance.
However, molecular spectroscopy can analyze the mass of molecules or fragments and including different elements and isotopes present in compounds.
The height of each peak represents the percentage abundance of the isotope of each element in the compound or molecule.
EXAMPLE.
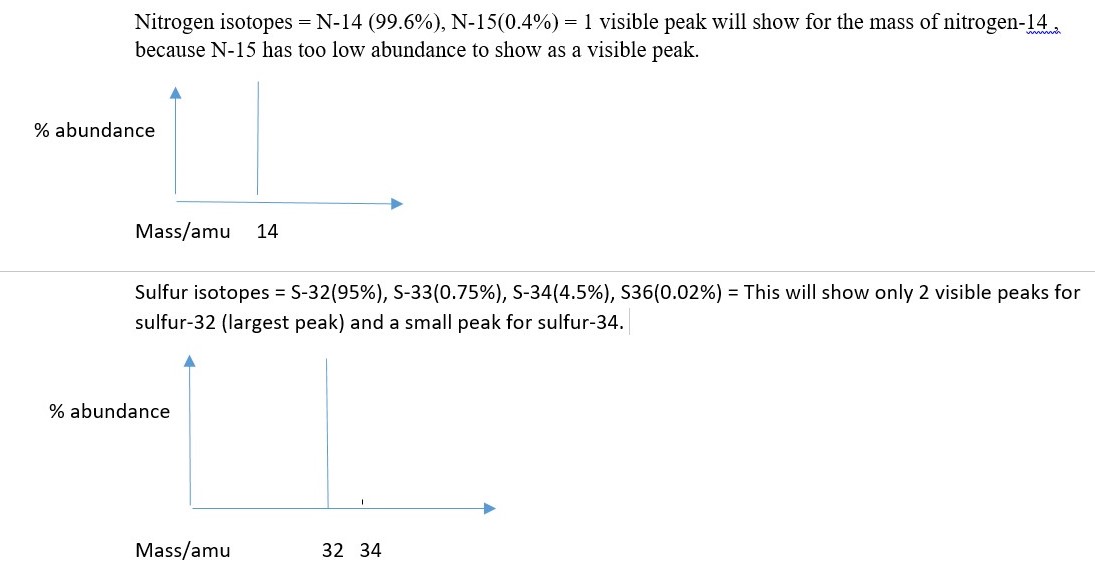
Carbon isotopes: Carbon-12(98.9%), Carbon-13 (1.10%) Carbon-14 (0.0003% not visible as peak) = therefore carbon shows only 2 visible peaks.
TYPES OF MOLECULAR MASS SPECTROSCOPY
There are several types or ways of measuring mass spectroscopy of molecules. Most of the methods are names after the way by which the sample initially processed before reaching the mass detector. The most common used in organic chemistry labs is gas chromatography mass spectroscopy [GC-MS].
GAS CHROMATOGRAPHY-MS
Sample solution injected is vaporized into molecules and bombarded with high energy electrons. These electrons can knock out a valence electron in an atom or an electron in a bond to form a molecular ion which is usually a RADICAL CATION.
MS process of a typical molecule.
The molecule is bombarded with high energy electrons which knock out an electron in the molecule to create a molecular ion.
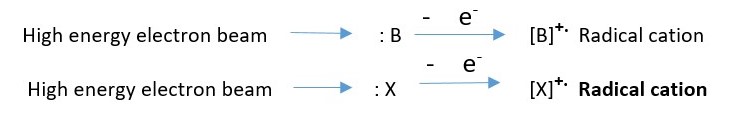
This shows that an electron can be removed from either X or B to form the molecular ion radical cation if either X or B has lone pair electron.
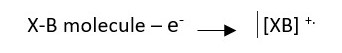
However, when no lone pairs are present electron is removed from a bond to form the molecular ion.
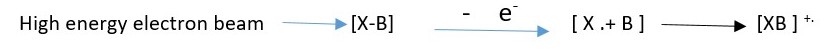
Depending on the structure of the original compound, the molecular ion radical cation may break apart into fragments, as it moves in the system and form cations and radicals, anions or neutral species.
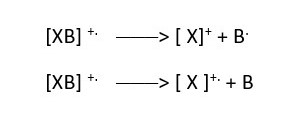
[B or B. will not record any mass value in mass spectrometry because THEY do not have + charge.]
The neutral species can lose an electron to form a CATION or a RADICAL cation.
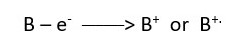
[will record a mass value on the mass spectrometry because it has + charge. ]
[B+ will record a mass value on the mass spectrometry because it has + charge.]
Radical cation can gain an electron to form an anion.
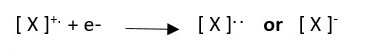
This will not record any mass in GC-MS because it does not have positive charge.
IMPORTANT NOTE:
A neutral atom loses an electron to form a + charge [cation] but a molecule loses a single electron to form a radical cation [.+] as the MOLECULAR ION.
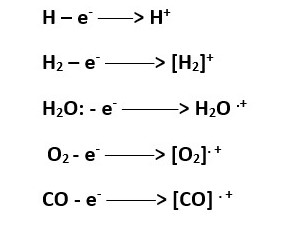
[Positive charge is required to record a mass value in mass spectroscopy.]
If an atom gains an electron, it gets a full negative charge, but a neutral molecule gains a single electron to form a radical.
Fragmentations and molecular weight
Not all molecular ions of the original molecule reach at the detector, but some tend to molecules break up into pieces of fragments of ions and radicals. The detector in GC-MS can only measure the mass of positively charged species. Therefore, it records only the mass of CATIONS and RADICAL CATIONS.
This is used to find the formula mass of the molecule being analyzed.
Height or intensity of peak = abundance of that positive ion of the Molecule or fragment or element reaching the detector.
Number of carbons in a molecule can be calculated from the intensity of the height of the C-13 abundance peak.
NUMBER OF CARBONS IN MOLECULE = (height intensity of [M+]+ peak) / 1.1
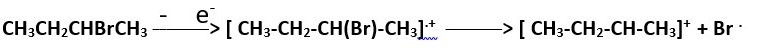
The Molecular ion [ CH3-CH2-CH(Br)-CH3].+ may form from when high energy electrons knocks out an electron from the atom that has lone pair electrons; the Bromine in this case.
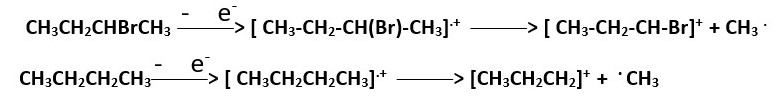
In this case high energy electrons knock out an electron from a C-H or C-C bond to form the radical cation Molecular ion [ CH3CH2CH2CH3].+ . In this state we have an empty sp3 orbital overlap with s-orbital electron cloud of hydrogen or sp3 orbital electron overlap with H+ or sp3 electron cloud of one carbon overlap with empty sp3 orbital of a nearby carbon atom.
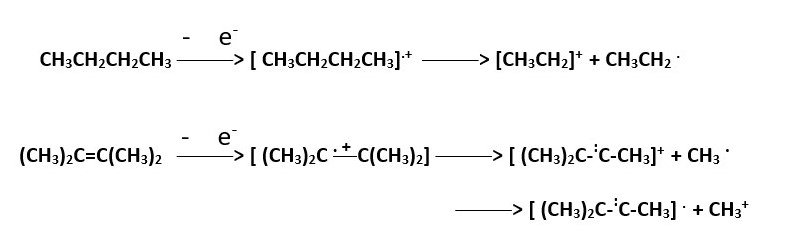
The allylic carbon stability makes it a good position for fragmentation to occur because the resultant cation species are stabilized by resonance and usually appears as a prominent BASE peak.
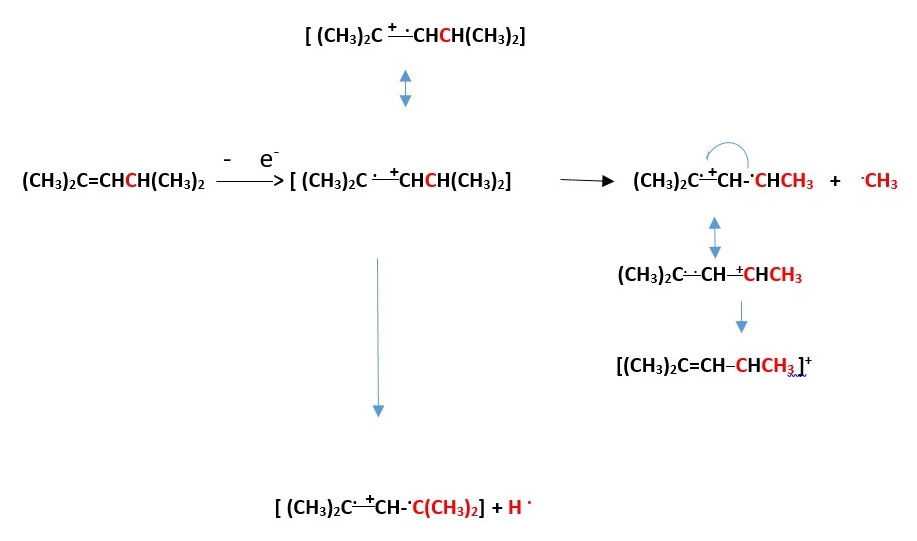
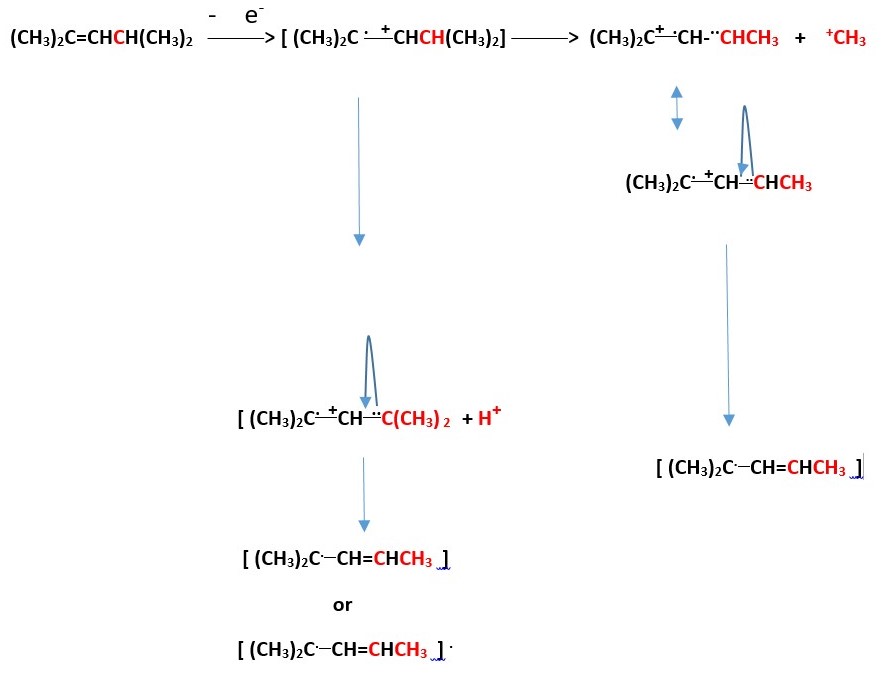
There is a tricyclic cation species that can form from the above process. Write the mechanism.
Calculate the formal charge on all the carbons in the final steps of the above processes.
The process below occurs to lesser extent compared to the above because the fragmentation forms high energy or less stable species +CH3 and H+ . A radical cation breaking up into two cations is a high energy process.
Resonance hybrid of molecular ion radical cation of benzene
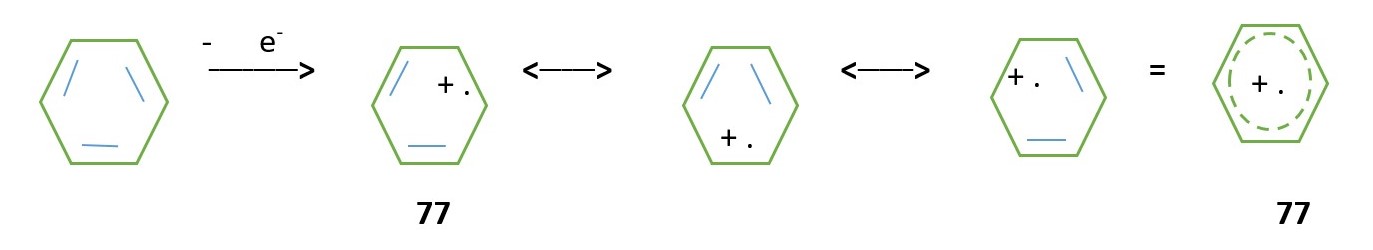
[sp2 molecular orbitals and calculation of formal charges on each carbon [add to find total charge on species].
TRY:
Write the mechanism for some of the likely transformations and fragmentations of the molecular ions of these molecules in GC-MS.
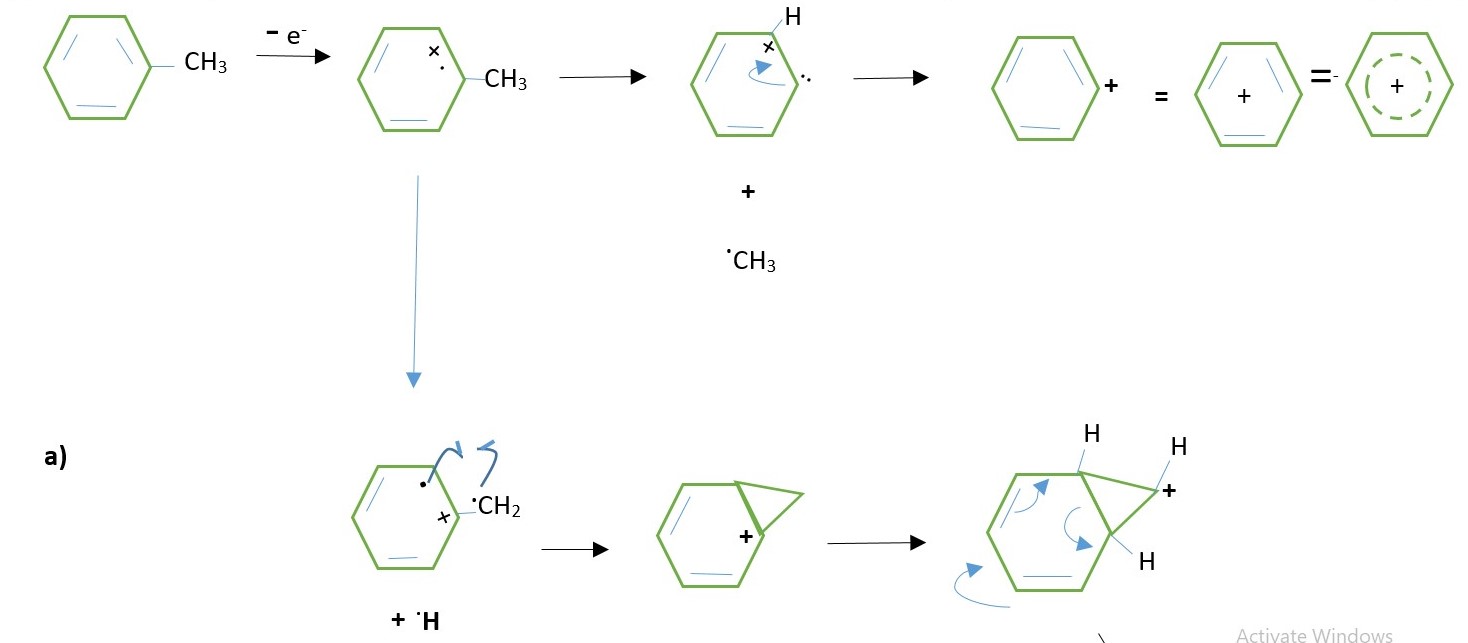
TOLUENE MOLECULAR ION IN GC-MS
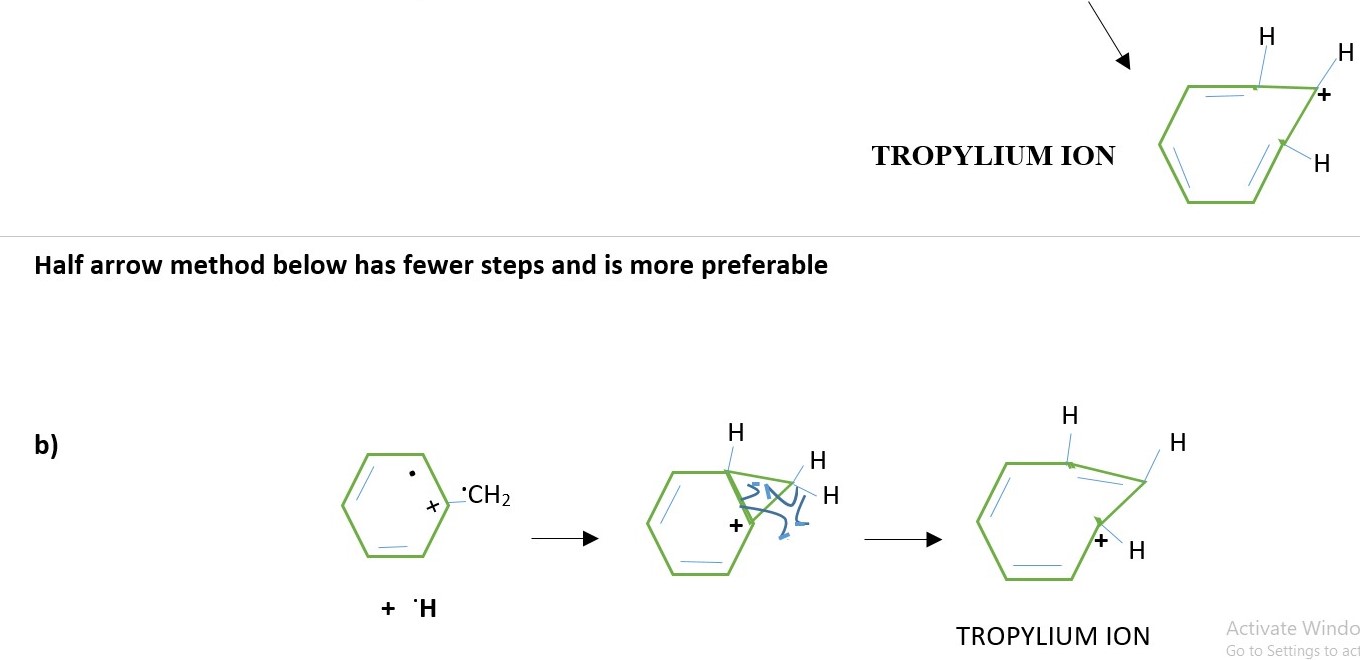
Half arrow method below has fewer steps and is more preferable
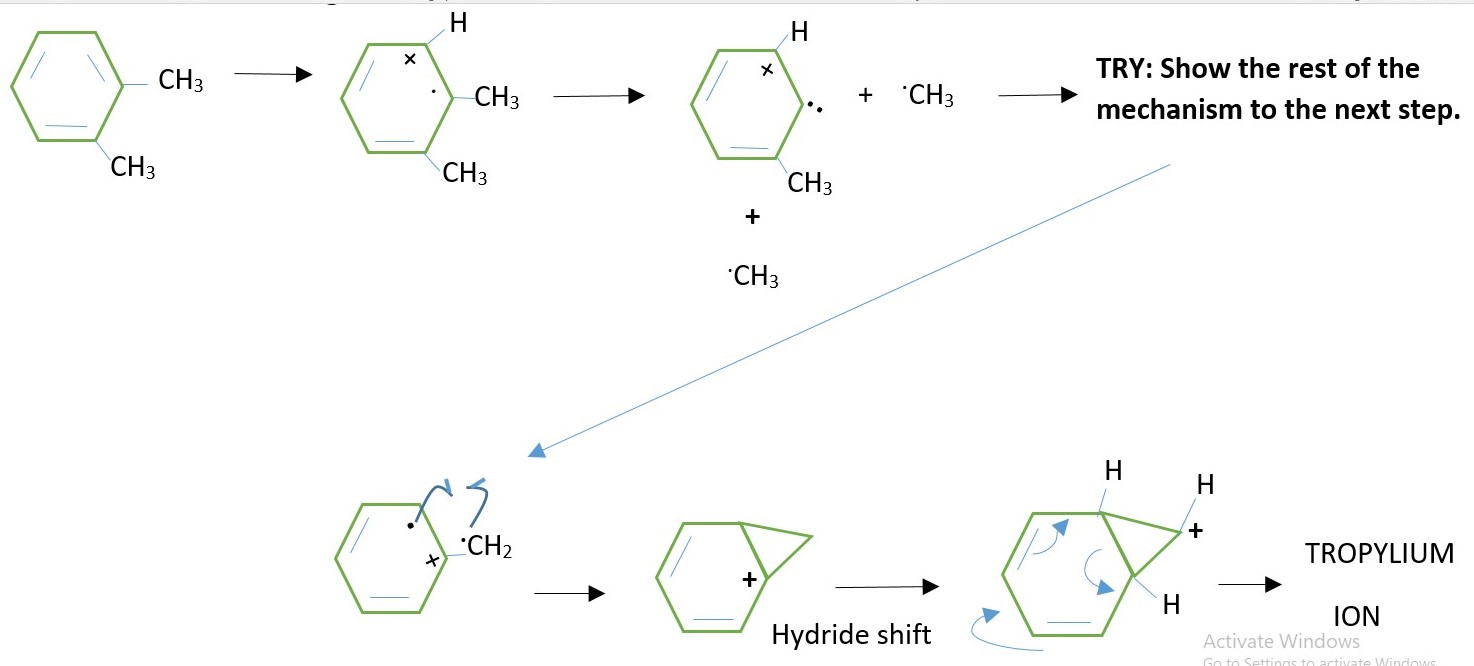
XYLENE molecular ion in GC-MS.
Fragmentation of molecular ion can also lead to formation of neutral molecules such as water, methane, ethene or alcohols instead of cations or radicals.
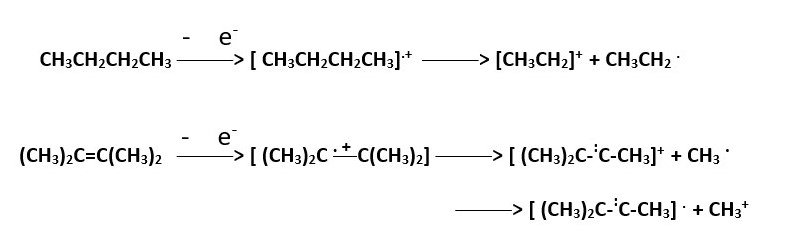
EXAMPLE:
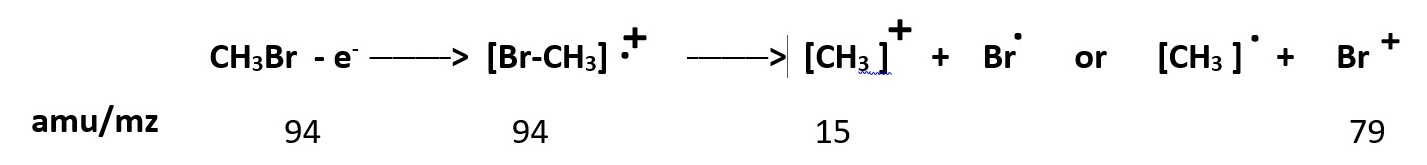
Theoretical representation of processes involving GC / Mass spectroscopy of Methyl bromide or Bromomethane [CH3Br] is illustrated below.
Fragmentations:
Molecular ions or fragments achieve stability by preferentially removing electrons from higher energy orbitals or bonds such as Π – bonds than sigma bonds or free lone electron pairs in p-orbitals in valence shells of atoms e.g. N, Br, O ,S or P .
Electron ejection from lone pairs on Br to form radical cation = Molecular ion M+. which easily fragments into Br+ and .CH3
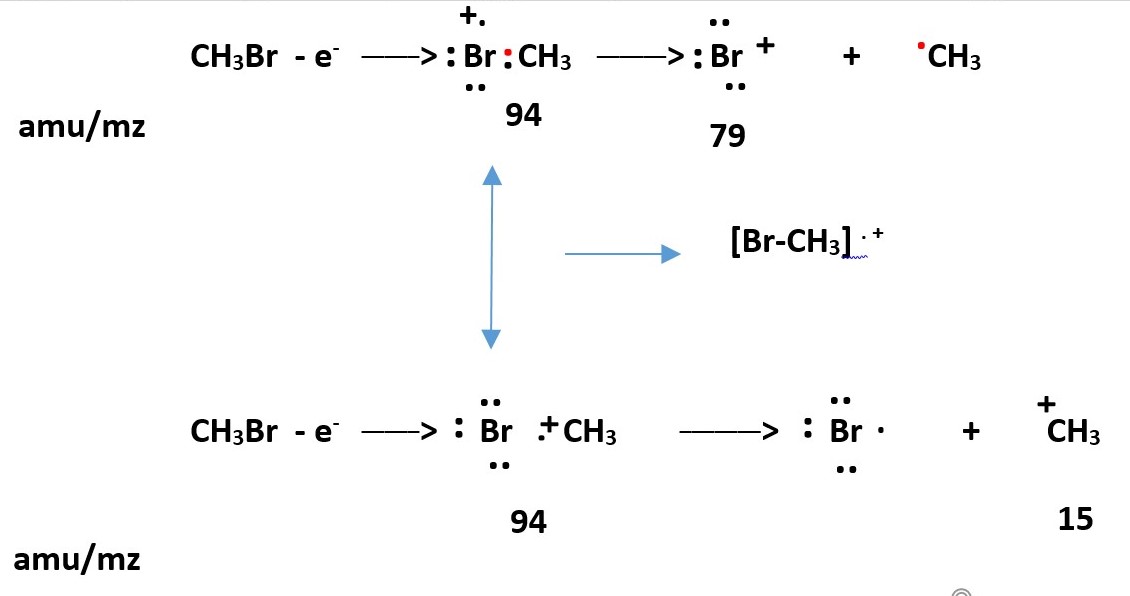
A bonding electron between Br-C ejection to form radical cation Molecular ion M+ . . Ejection of bonding electron require more energy than lone pair electron therefore fragments Br. and +CH3 occur in lesser amounts.
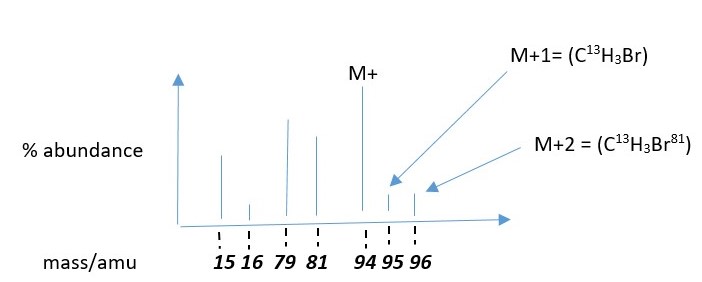
The diagram above is not drawn to scale just given for information purposes
Height of each peak represents the amount of that positive ion species that reaches the detector and this is related to its stability of the species which makes them form easily compared to others and easy it is to form that species in the mass spectrometry.
The above example shows that substantial amount of molecular ion remained to reach the detector showing mass 94 amu. This is because the molecular ion in this case is more stable than in other species formed from the fragmentation of this molecule.
The fragments are methyl ion [CH3+ ] peak at 15amu and Bromonium ion [Br+ ] peak at 79 amu. These are high energy species and will form in lesser amounts and show smaller peaks.
The peak at 16amu is due to C-13 isotope of the methyl ion C13H3 + . The peak at 96 amu is due to Bromine Isotope Br-81 for the molecular ion CH3Br81 +.
NITROGEN RULE IN GC-MS
If the mass of a molecule is an ODD NUMBER, then NITROGEN is present or ODD number of nitrogen atoms are present in the molecule.
If the mass of a molecule is an EVEN NUMBER, then,
-
there is NO nitrogen or there is even number of nitrogen atoms in the compound.
-
OXYGEN or sulfur is present or absent.