ORGANIC CHEMISTRY: STRUCTURE AND NOMENCLATURE
Structure and Nomenclature
The structure and formula of functional groups consist of a fixed number of elements and a specific chemical bonding . This could be single ,double or triple bond. Some common names and categories of functional groups in organic chemistry are listed below;
Functional groups
These are atoms or group of atoms in a molecule or compound that determines the way the molecule reacts with other molecules or atoms. There are several functional groups and their formula. Examples include alkanes RCH-, Alkanols RH2C-OH, Aldehydes RHC=O, Ketones R2C=O, Carboxylic acids RCOOH, Amides RCONH2, Esters RCOOR and more such as nitro R-NO2, amines RCNH2 etc.
The R group is represents an aliphatic group or an alkyl group. Examples includes a Methyl group -CH3 from parent name ; methane CH4. Therefore, methyl chloride has formula of CH3Cl. Similar examples are Ethyl groups CH3CH2– , Propyl groups CH3CH2CH2 -, Butyl groups CH3CH2CH2CH2 – and Pentyl groups CH3CH2CH2CH2CH2–
Alkanes:
The name ‘ane‘ is used to name all alkanes. General formula is CnH2n+2 where n is the number of carbon atoms in the compound. This is also the formula for saturated hydrocarbons. Examples include; Methane CH4, Ethane C2CH6 , Propane C3H8, Butane C4H10, Pentane C5H12, Hexane C6H14, Heptane C7H16, Octane C8H18, Nonane C9H20 , Decane C10H22, Undecane C11H24, Dodecane C12H26
You can get more information about structure and nomenclature by watching this video of structural isomerism of butanes and hexane.: click here
Alkenes
These have formula of CnH2n : These are unsaturated hydrocarbons with at one carbon-carbon double bond. The name of these compounds end with suffix ‘ene’. Examples include; Ethene C2H4, Propene C3H6 , Butene C4H8 , Pentene C5H10, Hexene C6H12, Heptene C7H14 , Octene C8H16 , Nonene C9H18
Alkynes
The formula of alkynes is given as CnH2n-2 . They are unsaturated hydrocarbons with at least one carbon-carbon triple bond. The suffix ‘yne’ replaces the ending of the ‘ane’ of the parent alkane. Examples include Ethyne C2H2, Propyne C3H4 , Butyne C4H6 , Pentyne C5H8, Hexyne C6H10, Heptyne C7H12 , Octyne C8H14 , Nonyne C9H16
You can get extra information about alkanes, alkenes and alkynes and other functional groups by watching the video on structural isomerism.
More detailed information on functional groups will be discussed later.
FUNCTIONAL GROUPS AND CHEMICAL NOMENCLATURE:
Besides alkanes, alkenes and alkynes, the presence of heteroatoms will introduce the rest of the functional groups in organic chemistry. Common heteroatoms are oxygen, nitrogen, halogens, sulfur and phosphorus.
IUPAC rank the functional groups with respect to oxidation and several other factors such including;
- Oxidation (oxidation number of carbon atom in group or number of oxygens, least number of hydrogens in the functional group.
- Acidity, resonance stabilization of the group or conjugate base or carbanion or carbocation, ease of formation of carbanion from alpha carbon; ease of undergoing substitutions and elimination reactions (derivatization).
- Number of oxygen atoms (or least number of non-protic hydrogens); presence of electron withdrawing groups; hybridization (sp>sp2>sp3);
- Stability of group or carbanion or carbocation formed.
These above factors will rank the functional groups in order of importance in determining the suffix name = [last name] of an organic compound.
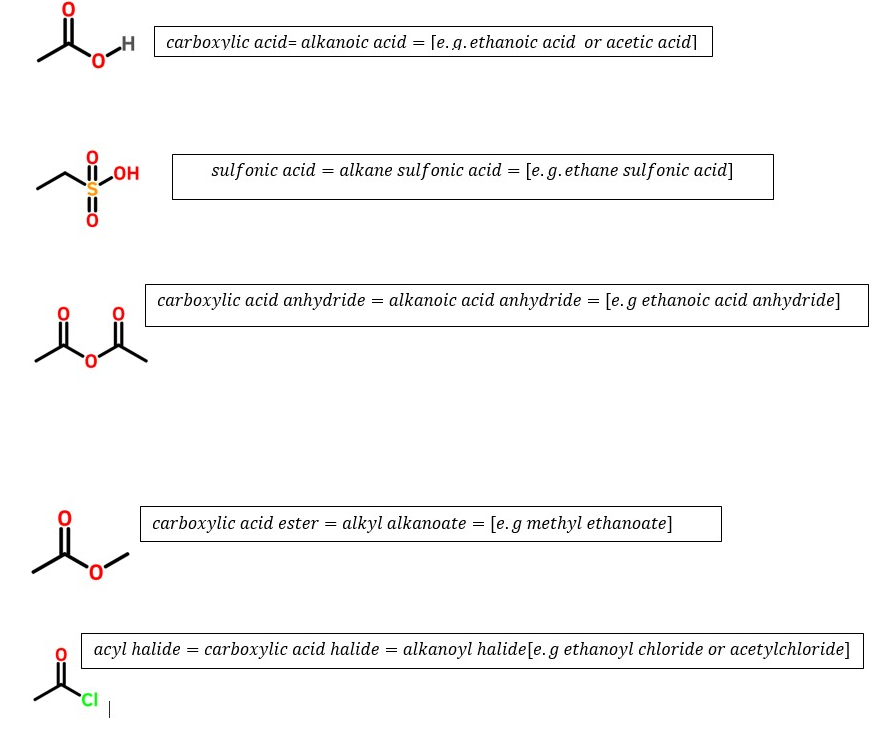
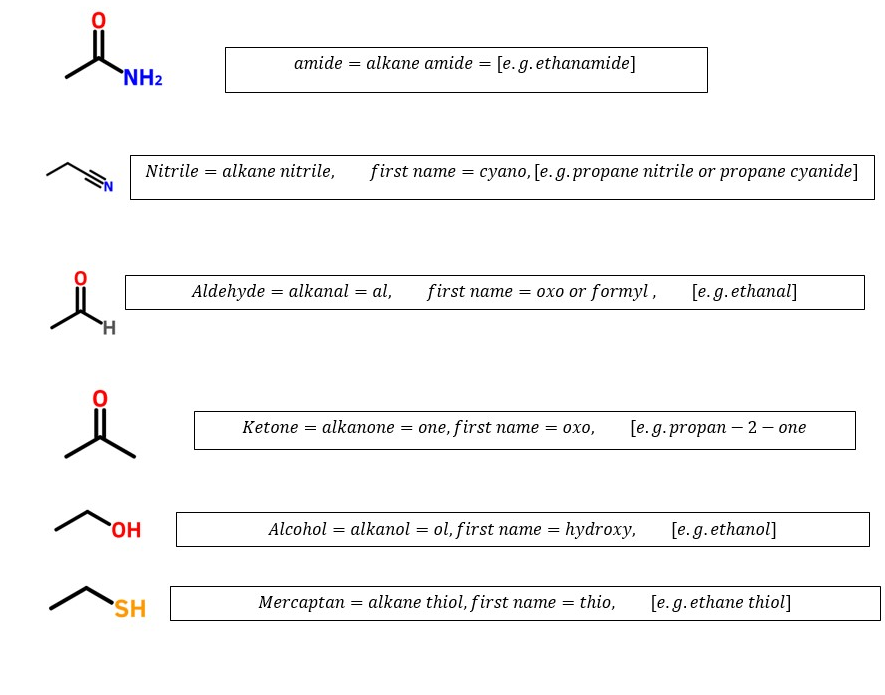
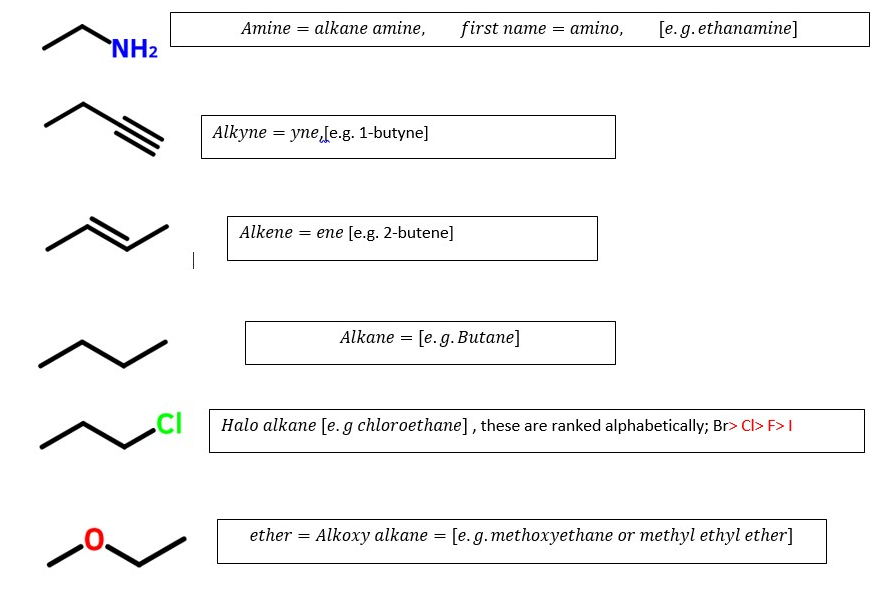
Carboxylic acids (-O=C-OH) > Sulfonic acids (CSO3H)> carboxylic acid anhydrides(-H2CO=C-O-C=OCH2-)> esters(-O=COCH3) > acyl halides(O=CCl)
Amides (O=CNH2>, nitriles(-C=N)> aldehydes (HC=O)> ketones(-CH2C=OCH3)
Alcohols(-C-OH) >Mercaptan (-C-SH)>amines(C-NH2) >alkynes(-C=C-)>alkenes(-CH=CH-)
Alkanes CH3-CH2CH3) >
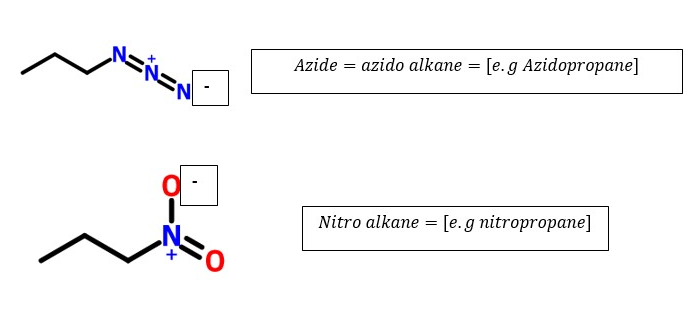
> alkyl halides (-H2C-Cl)>ethers(-H2C-O-CH2-) >azides or azido alkanes -H2C-N3> nitro groups(-H2C-NO2) [ These will occur as prefixes since they are ranked lower than alkanes]
RANKING ORDER
Ranking order of common functional groups that determines the last parent name of organic compounds: Highest determinant to least determinant.
Please watch all the videos on naming of alkanes [1, 2 and 3] and also structural isomerism to familiarize yourself on how to name organic compounds before you proceed to other functional groups.
Rules for naming:
- Start the with the name of the number of carbons as in alkanes but replace the ending “ane” with last name of the most important functional group in the compound. [e.g. Write the name of CH3COOH. The line structure is given below,
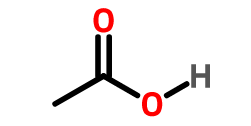
- CH3CH2OH naming is simple: [2 carbons =parent name is ethane, Functional group present =OH alcohol implies last name is alkanol = ol. Therefore, name of compound is Ethanol. CH3CH2SH name is also derived from 2 carbon parent ethane and functional group present as Sulfur = thiol or mercaptan therefore name is Ethyl mercaptan or Ethanethiol. CH3CH2-NO2 name is derived from 2 carbon parent name ethane, and functional group nitro NO2– is below alkane ranking so can only name it as a prefix “nitro”, therefore the name of the compound is nitroethane.
- Numbering is similar to alkanes and every other functional group is given a number at the carbon where it begins.
- Use dashes to separate numbers from names. [e.g. [ e.g BrCH2COOH = 2-Bromoethanoic acid, CH2=CHCOOH = 2-Propenoic acid], BrCH=CHCOOH is called 3-bromo-2-propenoic acid, BrCH=CH-C=C-COOH [this has 5 carbons in longest chain implies pentanoic acid], numbering and naming: Carbon#2 alkyne= yne , Carbon #4 alkene= ene ,number 5 has Bromine=bromo. Therefore, name will be 5-Bromopent-4-en-2-ynoic acid
- Use comas to separate numbers that represent the same substituent attached multiple times to carbons in the compound. Examples of this is shown in the video for naming alkanes.
- The carbon of highest ranked functional group in the compound should be included in counting the continuous carbon chain that determines the name of the compound. This applies even if it is not the longest chain. However, check to see if you can get the smallest number as possible for the main functional group and the other functional groups and substituents as well as the overall sum of the numbers used in the numbering. This is very important especially when the functional groups occur on the middle carbons. e.g., Write the name of the compound.
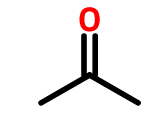
- CH3C=OCH3 [3 carbons =parent name is propane; functional group present is ketone=alkanone implies last name is “one“. Therefore, name of compound is propanone, however functional group C=O is at number # 2, implies proper name is 2-propanone or Propan-2-one, CH3C=OCH2CH(CH3)CH2COOH This contains a carboxylic acid functional group COOH (most important) so naming should end oic acid. The longest chain containing the COOH group is 6 carbons= hexanoic acid. Carbon number 3 is bearing a methyl substituent (CH3) and carbon number 5 is having a ketone C=O functional group implies first name use for ketone =oxo. Therefore, the name will be 3-methyl-5-oxohexanoic acid. The other example is writing the name of CH3C=OCH2CH=CHC=CH. The line structure is drawn below;
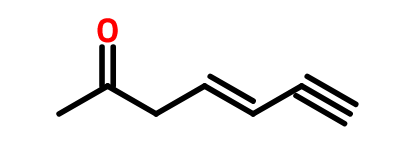
- The other example is finding the name of the compound shown below;
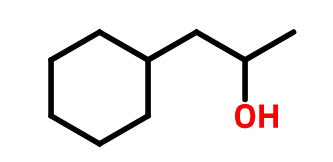
OTHER RULES
- If the number is 1, you can ignore number one in most cases. e.g., 1-ethanol or Ethan-1-ol is usually called Ethanol.
- Functional group only takes the last name if it ranks the highest in the compound otherwise only their first names are used. e.g., Ketone is alkanone or last name is “one”. However, if it is not the highest ranked then the first name is “oxo“. Alcohol OH group last name is alkanol =”ol”, but first name is hydroxy if it is not the highest ranked functional group in the compound. e.g., HOCH2CH2CH=O; [3 Carbon = parent name is propane, there are 2 functional groups Aldehyde CH=O alkanal = “al” and also there is alcohol OH group. However, aldehyde is more oxidized than alcohol, so it ranks higher. so, the name of the compound will be 3-hydroxypropanal]. HSCH2CH2OH is called 2-mercaptoethanol because the –OH ranks higher than –SH
- Besides the highest ranked functional group, the rest of the names are written in alphabetical order, but numbering can be different. e.g., alkene =ene comes before alkyne =yne but alkyne takes the smallest number when only both are present except in a symmetrical compound when alkene takes the smallest number.
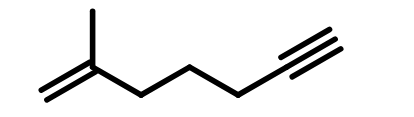
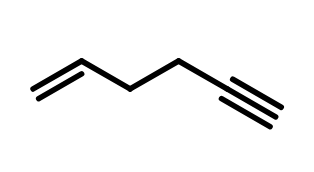
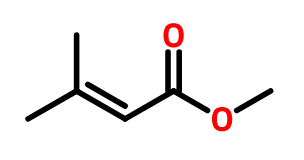
Therefore, the name will be Methyl 3-methyl-2-butenoate.
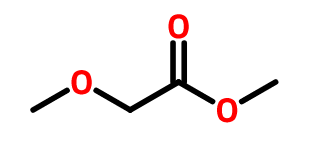
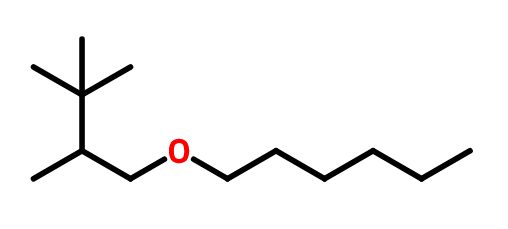
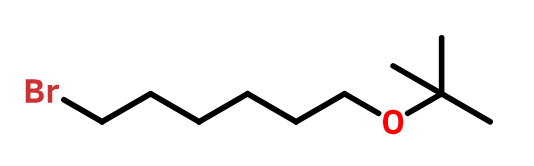
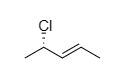
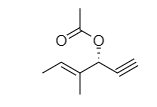
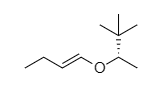
OTHER EXAMPLES :
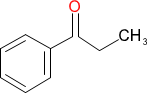
1-phenyl-1-propanone or 1-phenylpropan-1-one or 1-phenylpropanone
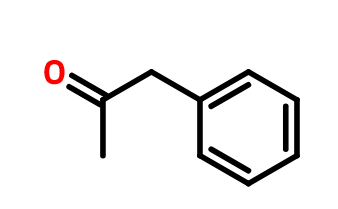
1-phenyl-2-propanone or 1-phenylpropan-2-one
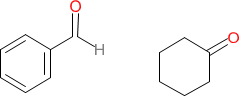
The first compound is benzaldehyde and the second compound is called cyclohexanone.
Practice naming these compounds below

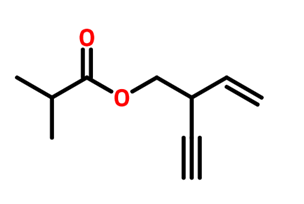
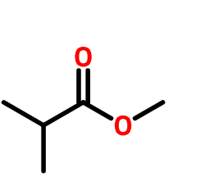
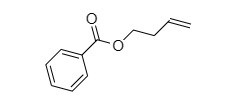
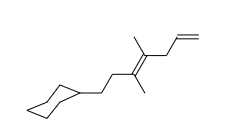
Video of structure and nomenclature of alkanes: click here


