ORGANIC CHEMISTRY: NMR spectroscopy
NMR: Nuclear Magnetic Resonance
This is radiation emitted by spinning protons in certain elements. Protons are made to spin under certain magnetic frequencies. The excited spinning protons fall back to ground state in a relaxation process by emitting a radiofrequency that can be detected and recorded to determine the position or chemical environment of the nucleus that emitted the radiation. The spectroscopy of the spinning nuclei is called NUCLEAR MAGNETIC RESONANCE or simply NMR.
Elements usually used for NMR spectroscopy include Carbon, Hydrogen, Nitrogen, Oxygen, Sulfur and Fluorine and Phosphorus. These have ½ nuclear spins or odd multiples of ½ spin.
TERMINOLOGY
Multiplicity: Number of signal peaks from a proton in a molecule.
Coupling: Interaction between two elements from spinning of the nuclei
Coupling constant: Number representing magnitude of coupling of two element nuclei spins.
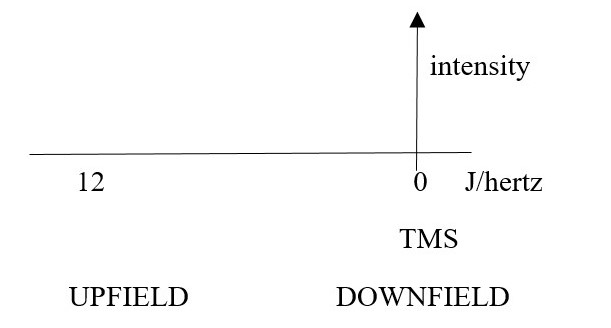
SHIFT: How exposed or shielded a nucleus has been to the external field. This gives the chemical shift number value for the signal position of the element. The peak position on x-axis in NMR spectrum is called a CHEMICAL SHIFT in hertz. [0 to 12].
Magnetic field strength X chemical shift (ppm) = chemical shift (Hertz).
The position of a peak signal is shifted UPFIELD to higher wavenumbers in the spectrum, if a proton is more exposed to the external magnetic field due to de-shielding chemical environment such as
-
Presence of electronegative atom or group.
-
Presence of de-shielding hybridization such as sp or sp2 carbon
Exposed or de-shielded nuclei appear as signals in HIGHER wavenumbers up-shift or up-field in the spectrum.
The position of a peak signal is shifted DOWNFIELD to lower wavenumbers in the spectrum, if a proton is less exposed to the external magnetic field by a shielding electron cloud in the chemical environment such as
-
Presence of bulky less electronegative atom or group.
-
Presence of BULKY sp3 hybridized carbon bonds, atoms or groups.
-
Presence of pi bond electron cloud
Shielded nuclei appear as signals with lower wavenumbers down-shift or down-field in the spectrum.
De-shielding of electron cloud can be caused by
-
highly electronegative such as HALOGENS, OXYGEN
-
high s-character hybrid molecular orbitals such as sp, sp2 presence in the molecule.
-
Positive effective charge on the molecule.
SIGNAL INTEGRATION
SIGNAL INTEGRATION: number of protons per signal peak in NMR spectrum.
Integral sign used and can be measured from the computer or manually from the relative RATIOS of the vertical lengths or relative ratios of the AREAS of each integral.
E.g. A peak signal integral from one two protons will be twice the peak integral of a signal caused by only one proton in the same molecule.
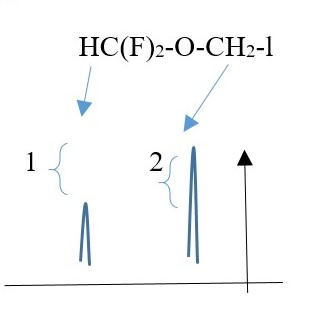
The height intensity or intensity of a peak signal relates to the number of protons causing the signal to be produced.
E.g. Singlet peak from a single proton is shorter peak compared to the height intensity of a singlet peak caused by two, three, four, five or six protons.
TYPES OF SIGNAL PEAKS IN PROTON NMR
Proton or protons would give a single peak signal (SINGLET) if
-
because the chemical environment is the same for each proton in each compound. Meaning the protons are the same or EQUIVALENT or HOMOTOPIC.
-
They are isolated by more than 3 or 4 bonds away
-
Isolated attached to a different element other than carbon.
EXAMPLE
Protons in the structures below will show as single peak or SINGLET in the NMR spectrum because they have no adjacent protons of different chemical environment that will split their peak signals. However, each singlet would appear at a different chemical shift and integrals will equal the number of protons for the singlet peak.
CH4 , CH3-C(Br)=O , -A-CH2-A- , H2C=O , -COOH , CH3-BrC=CBr-CH3 , -A-C(H)=O ,
, A-NH2
The following below have more than one singlet peaks
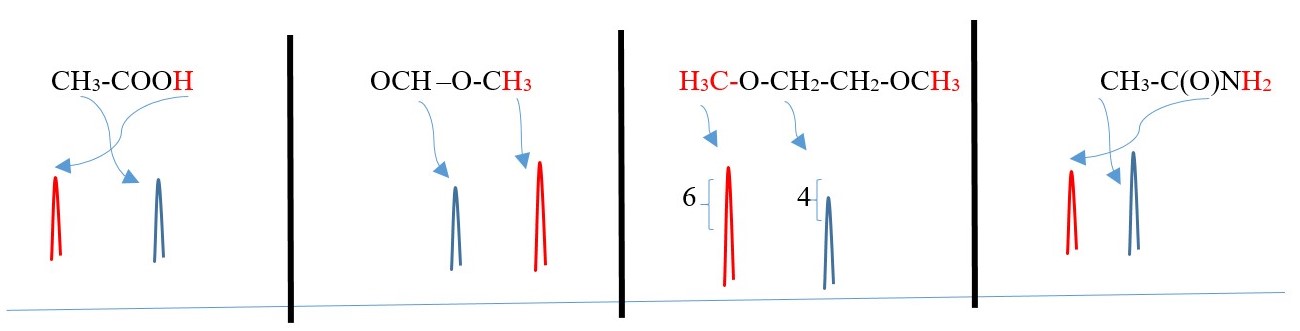
Two or more DIFFERENT singlet separate peaks will occur from protons on carbons that cannot split each other because they are attached to carbons that are farther away from each other in the same compound.
SIGNAL COUPLING
Proton in a different chemical environment has a different chemical shift and therefore a different spin frequency relaxation magnetic effect.
Magnetic effect from spin relaxation of a proton in a different chemical environment is different can interact with the magnetic effect of a proton spin on adjacent carbon of different chemical environment. This interaction called SPIN Coupling causes the splitting of the total magnetic effect of the proton spin relaxation into multiples of signal peaks in the NMR spectrum. Number of peak signals corresponds to the number of protons on the adjacent carbon of different chemical environment.
There is NO splitting of signals of protons of the same chemical environment.; all proton spin relaxation occurs in one relaxation mode or direction. Corresponding to only ONE peak signal.
Splitting of proton signals usually occur if the two protons are separated by 3 bonds. This is the number
of bond separation that gives clear and meaningful splitting of signal speaks in proton NMR spectrum.
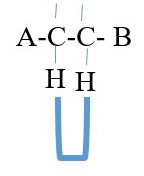
A is same as B = NO coupling or splitting of peaks = single peak = singlet peak only
A is not B = COUPLING = SPLITTING of signal peak into Doublet of doublets
MULTIPLICITY
NUMBER OF PEAKS EXPECTED FROM A PROTON IN A MOLECULE = MULTIPLICITY = N+ 1 [ where N = Number of nonequivalent protons on adjacent carbons that can split the peak signal. ]
Nonequivalent protons are protons on adjacent carbon with a different chemical environment.
A proton will show multiple peaks or MULTIPLETS when its signal peak is split by more than two nearby protons.
Multiplets can be a QUARTET, PENTET, SEXTET, HEPTET or more.
E.g. All these structures [ A-CH3 , A-CH2-CH2-A, CH4 , CH3-CH3 , Benzene , cyclohexane ] would give a SINGLET peak at room temperature because all the protons are equivalent or homotopic and therefore N=0 . Multiplicity = 1 = singlet.
Molecules with more than one singlet peaks have protons located at positions where their signals cannot be split by other proton signals.
Coupling can occur if protons are separated by carbons of different hybridizations [sp, sp2 or sp3]. However, this is only usually observed using a high resolution instrument [e.g. 400MHz NMR].
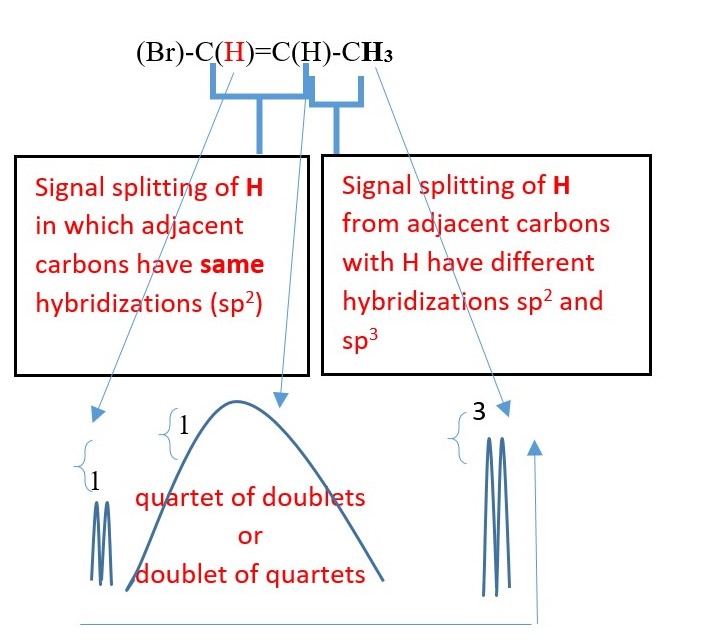
EXAMPLE
H-(Cl)C=C(Br)-H
Signal splitting due coupling through double bond. Cis / trans coupling will have different coupling constants.
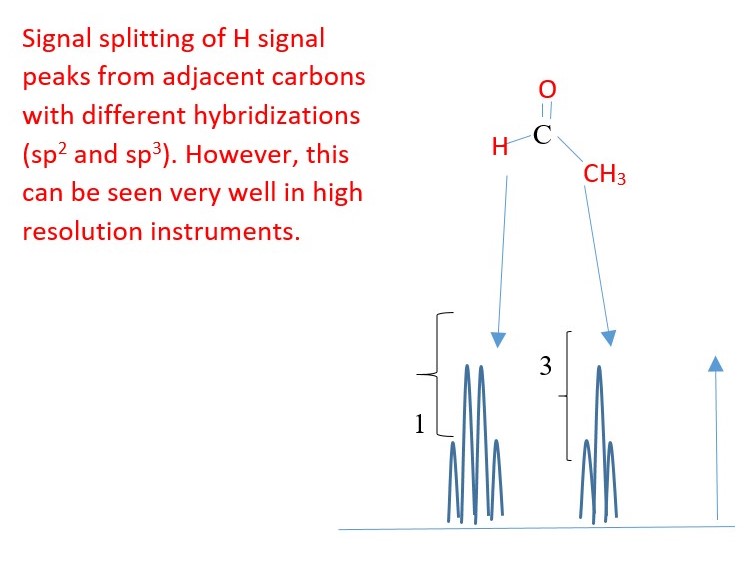
Proton bonded to a DIFFERENT element can split with proton bonded to carbon but exchange of these protons between the molecules and water impurities will remove the coupling splitting and cause the hetero protons to appear as singlets on low resolution instruments.
A HIGH resolution spectrum will easily show the signal peak splitting of hetero-H due to coupling with C-H protons. E.g.
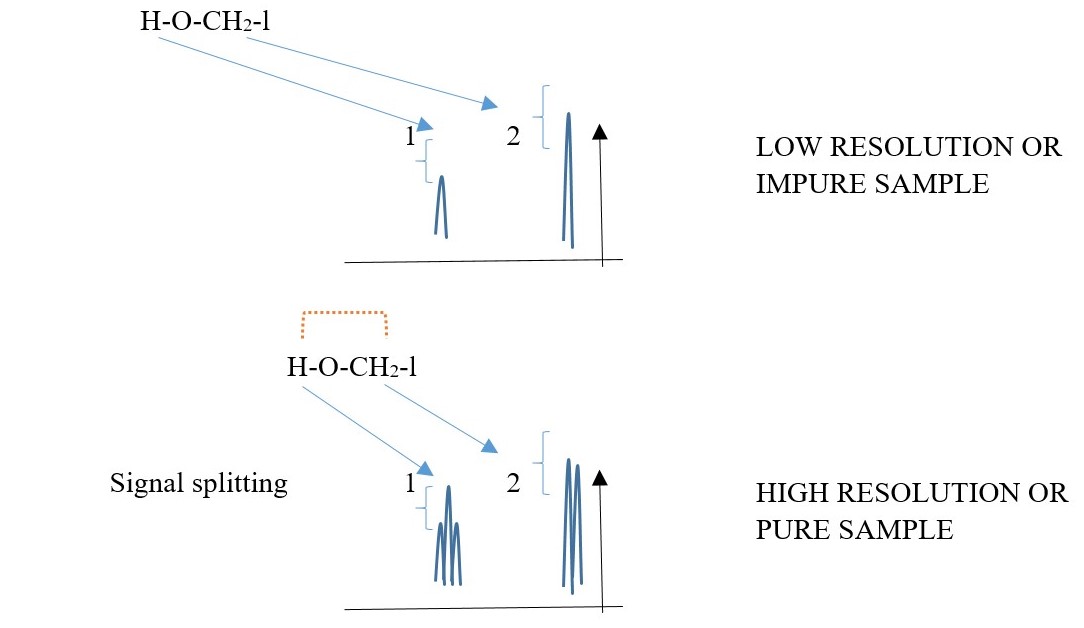
Predict the NMR spectra of the following compounds
N-Methylacetamide, Methanol, Methanal, Ethanal, Acetone, Acetic acid, H2C=N-H, HC = P-H
Example 1,
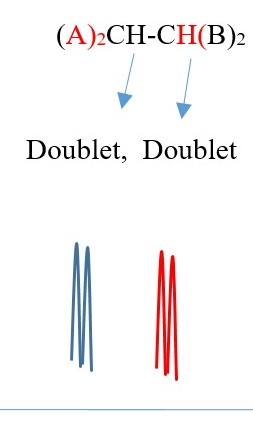
TWO protons near A chemical environment are equivalent and homotopic and interact with the signal of the single non-equivalent proton [N=1] on adjacent carbon near B chemical environment to give a multiplicity peak signal of [ N+ 1 = 1+1 = 2] = DOUBLET. However, the proton near B also sees a single non-equivalent proton near A chemical environment and the interaction splits the signal peak into a multiplicity peak signal of [N+ 1 = 1+1 = 2] = DOUBLET of a different chemical shift.
Example 2
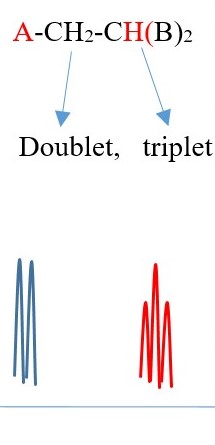
TWO protons near A chemical environment are equivalent and homotopic and interact with the signal of the single non-equivalent proton [N=1] near B chemical environment to give a a multiplicity peak signal of [N+ 1 = 1+1 = 2] = DOUBLET. However, the proton near B also sees two non-equivalent protons [N=2] near A chemical environment and the interaction splits the signal peak into a multiplicity peak signal of [N+ 1 = 2+1 = 3] = TRIPLET with a different chemical shift due difference in the chemical environment.
The spectrum will have two triplets of the same height but different chemical shifts because A is different from B . If A=B then all the protons will be homotopic and will show only one singlet.
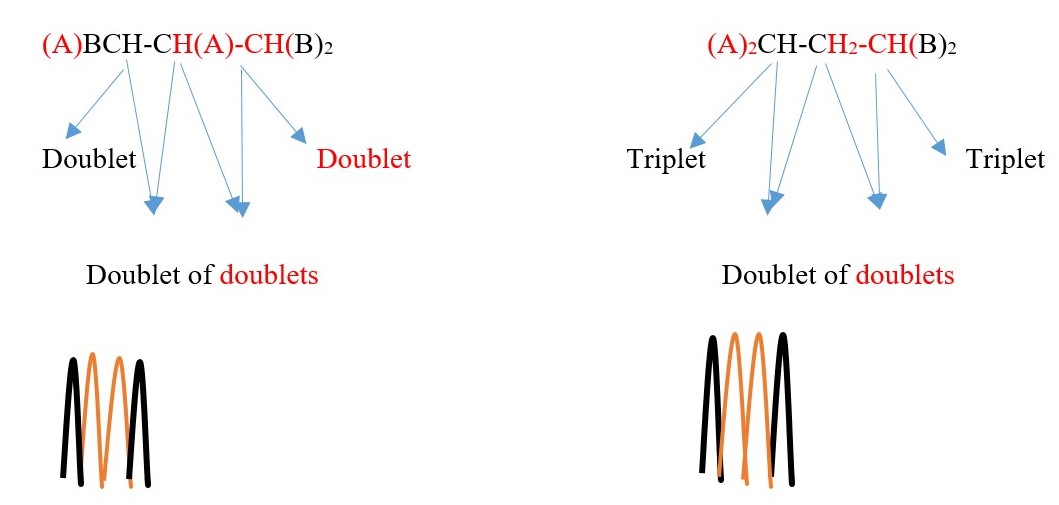
Example 3 ,
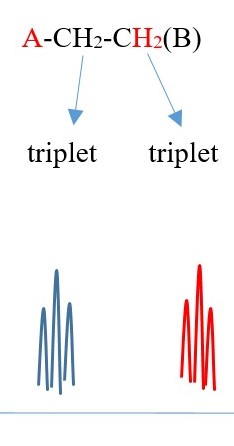
TWO protons near A chemical environment are equivalent and homotopic and interact with the signal of the TWO non-equivalent protons [N=2] on adjacent carbon near B of different chemical environment to give a multiplicity peak signal of N+ 1 = 2+1 = 3= TRIPLET. However, the 2 protons near B sees a two non-equivalent protons [N=2] on adjacent carbon near A chemical environment and the interaction splits the signal peak into another multiplicity peak signal of [N+ 1 = 2+1 = 3 ]= TRIPLET of a different chemical shift due to difference in chemical environment A and B.
Example 4 ,
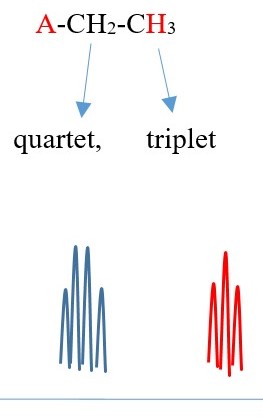
TWO protons near A chemical environment are equivalent and homotopic and interact with the signal of the THREE non-equivalent protons [N=3] on adjacent terminal carbon of different chemical environment to give a multiplicity peak signal of [N+ 1 = 3+1 = 4 ] = QUARTET . However, the THREE terminal protons are also homotopic or equivalent and also sees TWO non-equivalent protons [N=2 ] on adjacent carbon near A chemical environment and the interaction splits the signal peak into a multiplicity peak signal of [N+ 1 = 2+1 = 3]= TRIPLET of a different chemical shift.
Each proton gives a triplet because chemical environment imposed by A is different from B. Each proton sees two protons in a different chemical environment. Total result will be two different triplets side by side.
SYMMETRY
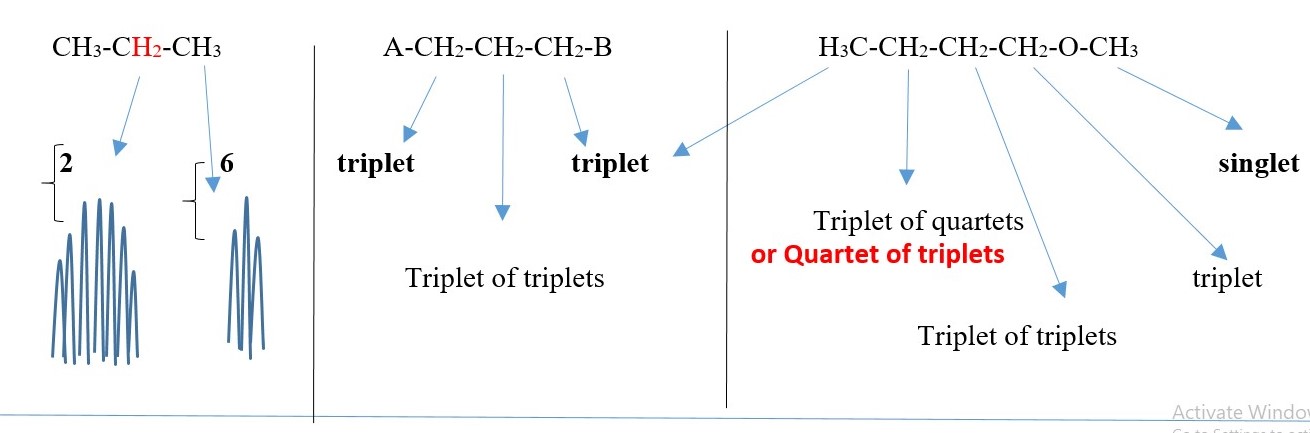
SYMMETRIC COMPOUNDS produce peak signals corresponding to half structure, but the integrals should be the total number of protons in the molecule. Therefore, all structures below would give a singlet peak but the integral would be the total number of protons in the compound.
-CH2-CH2– , CH3-CH3, (CH3)2C=O , CH3-O-CH3, A-CH2-CH2-A, A-CHB-CHB-A
[ A or B is different element or functional group]
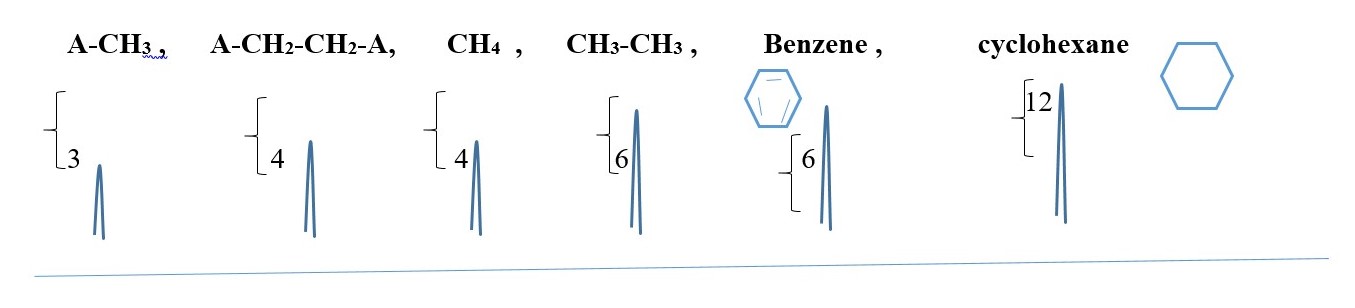
This is just to show the number integrals values and the peak SINGLET for each case but the chemical shift values will differ for all the peaks.
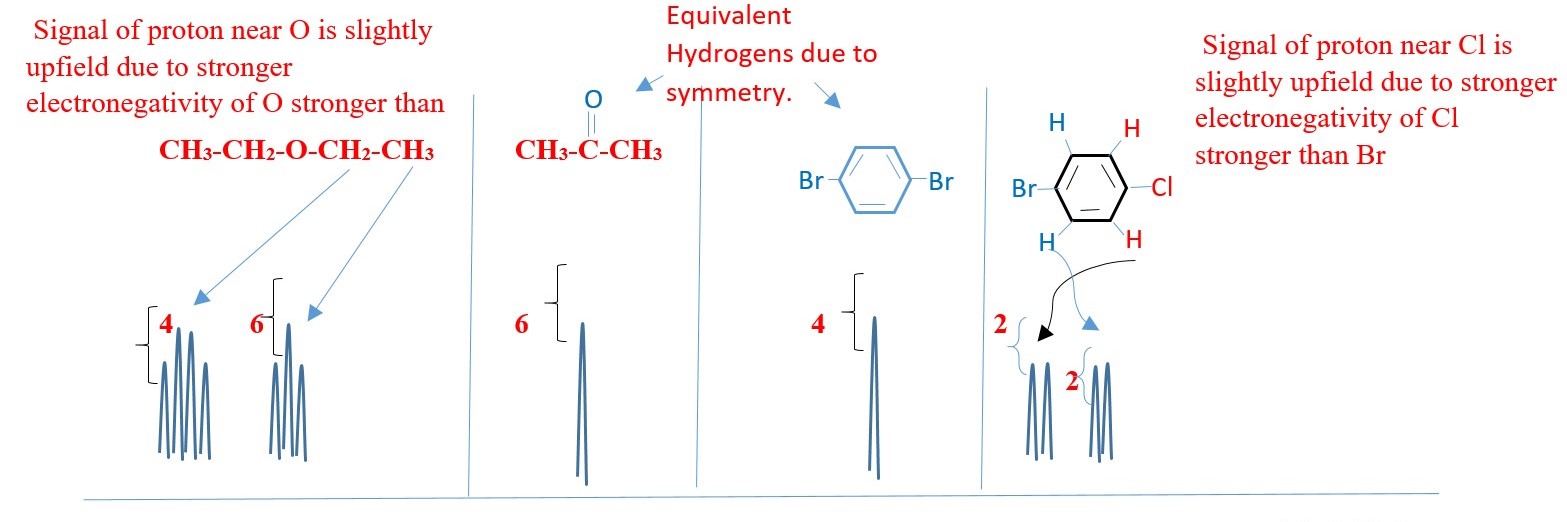
COUPLING CONSTANTS AND CALCULATION
Measured distance (Jhh /hertz) of the peak of one proton signal to the peak of the other coupled signal.
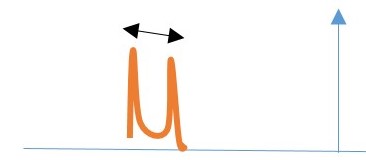
This can be calculated manually or by using a computer software
Hydrogens three bonds apart have clear coupling and coupling constants.
The 5 protons in BENZYL or PHENYL group can appear as a singlet on a low-resolution NMR. However, coupling can also occur in protons from 4 or more bonds away as in BENZYL or PHENYL compounds.
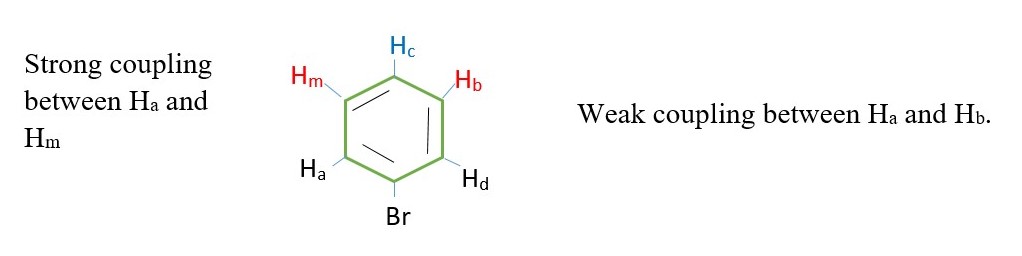
The inner protons will have their signal split by surrounding protons such that the spectrum will be much more complex.
E.g. Hm– Ha coupling, while Hm– Hc coupling, Hb – Hc , Hb – Hd etc.
Coupling constants must be measured the same way throughout an experiment for accurate and precise references.
Coupling constant reveals, the type of protons and how close they are from each other. A HIGH value for a doublet would mean good coupling protons next to each other on different carbons in different chemical environments.
The nature of the substituent on the benzene can affect the value of the coupling constants. The coupling constants between the peaks in all the above are the same value. Two coupled protons will have the same coupling constants whether one is a triplet, or doublets or quartet.
COMPLEX MULTIPLETS (non-conventional splitting)
Complex Multiplets are signals from central protons surrounded by two or more non-equivalent protons on adjacent carbons. In this case, it is important to ALWAYS USE ONLY THE CENTRAL REFERENCE PROTON to determine the pattern of the signal splitting.
The nature of a complex multiplet depends on value of coupling constants of the nmr spectrum from the experiment.
E.g. for a central proton with two adjacent –C-H protons, -XCH-CH-CHZ-
1) determine the split signal from MID-proton versus the LEFT side proton on adjacent carbon that is -XCH-CH,
2) then determine the signal splitting of same MID-proton versus the RIGHT side proton on adjacent carbon that is -CH-CHZ.
3) Then find which has highest coupling constant.
4) Write the name beginning with the highest coupling constant, and end with the least.
E.g. If we expect a proton signal to be DOUBLET OF TRIPLET or TRIPLET OF DOUBLETS, the experiment will eliminate one of the outcomes. The signal with LARGER value of coupling constant determines the first name.
Therefore, if the complex multiplet has DOUBLET with larger coupling constant than TRIPLET, then the name will be DOUBLET OF TRIPLETS and vice versa if TRIPLET has larger value coupling constant [ TRIPLET OF DOUBLETS].
A DOUBLET OF DOUBLETS occur when one doublet has a higher coupling constant than the other from the same central proton.
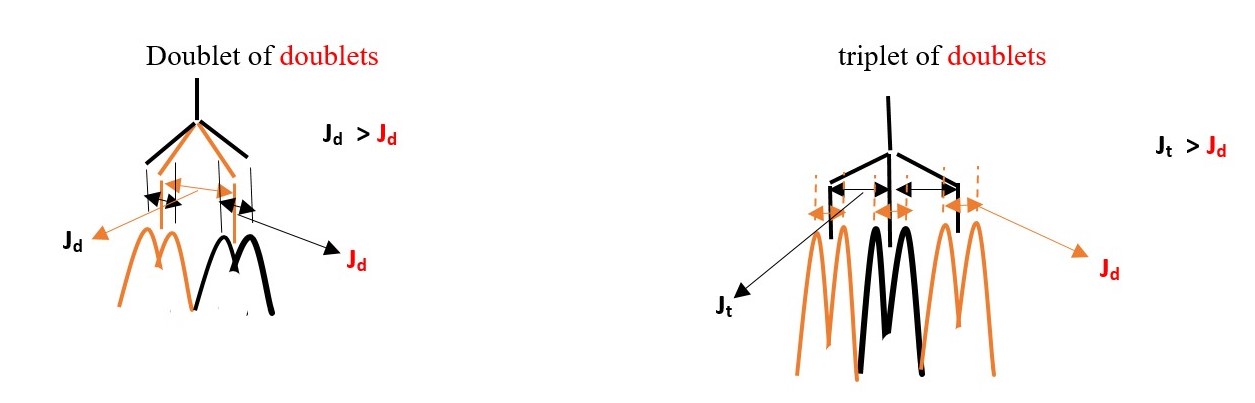
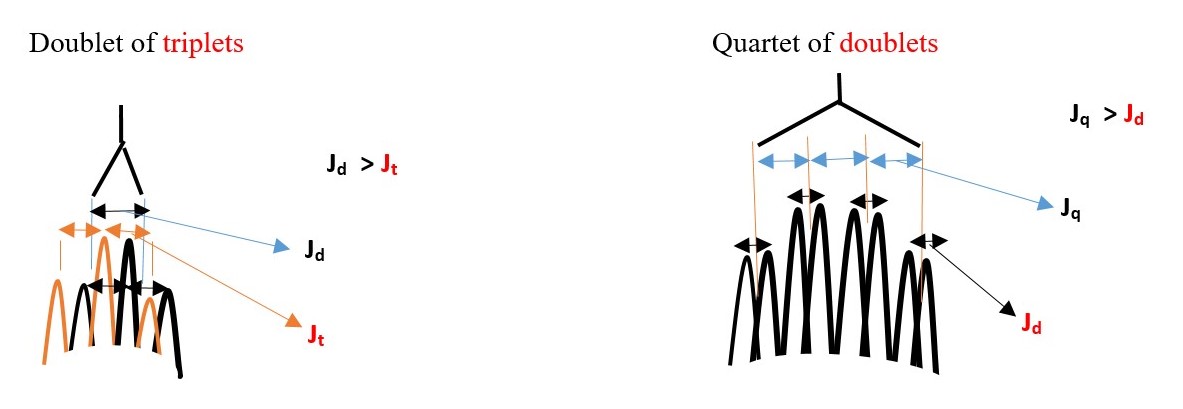
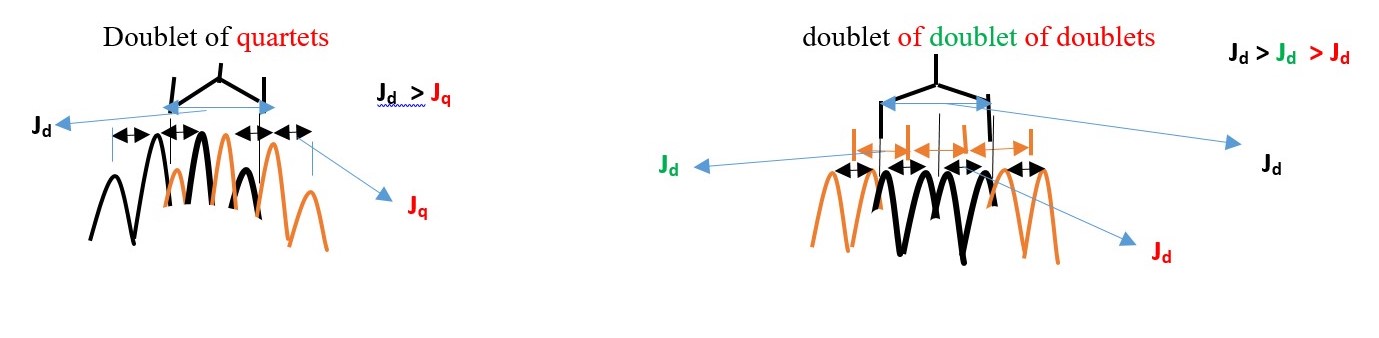
Some expected signal peaks ;
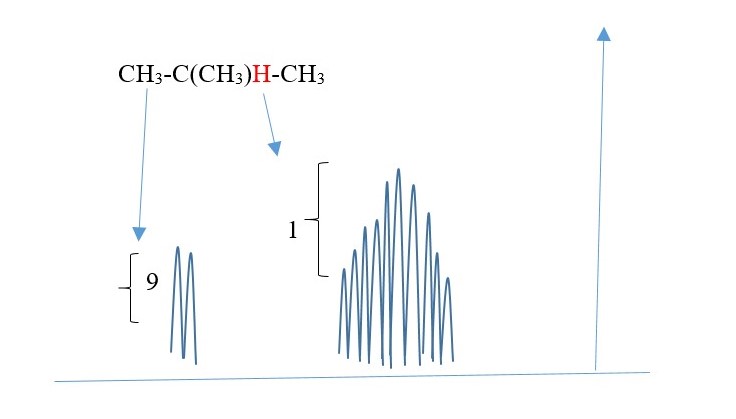
Each H in -CH3 is equivalent(homotopic) equal distance from each other and from H (central). Therefore, all -CH3 [N=1 for one H] show as a one DOUBLET [since peak that integrates to 9 protons.
However, H is surrounded by nine protons different environment from Central H. therefore N=9 for central H ; Multiplicity = 10 peaks (multiplex) for Central H.
EXAMPLE
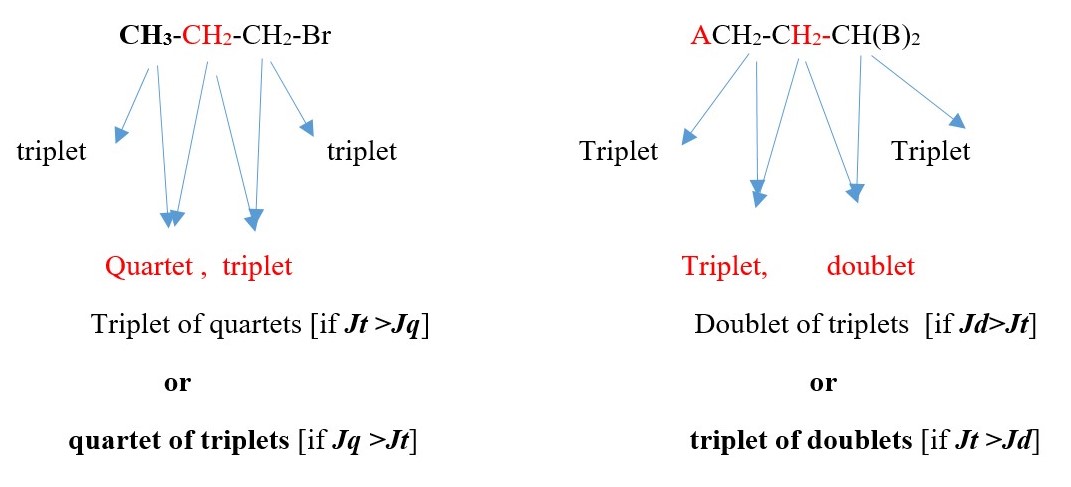
Find expected NMR signal peak structure of the central -C-H proton in ACH2-CH(CH3)-CHB2
The proton signal peak of the central carbon –C-H will be a complex multiplet but will be found by signal interactions with the adjacent protons. The following signals will occur with reference to the central -C-H proton signal interaction with its adjacent protons.
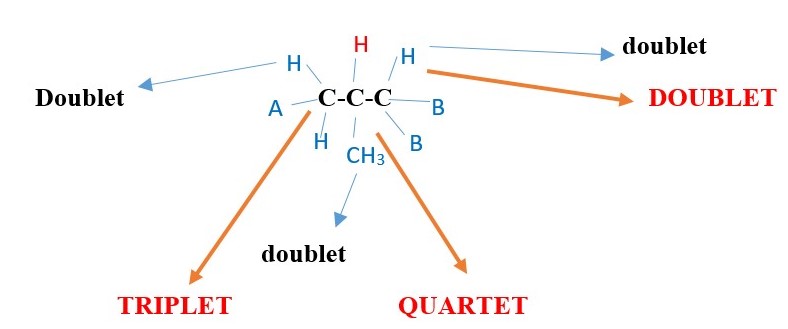
-
The central Proton H peak signal will be split by the left side protons into triplet
-
The central Proton H peak signal will be split by the RIGHT side proton into Doublet
-
The central Proton H peak signal will be split by the –CH3 adjacent protons into QUARTET
-
Therefore, central H signal can be:
Doublet of triplet of quartets. [If Jd >Jt >Jq ] or
Triplet of doublet of quartets. [If Jt >Jd >Jq ] or
Quartet of doublet of triplets. [If Jq >Jd >Jt ] or
other possibilities. in this case but the actual signal can even be more complex than the above three because the central proton is chiral and can split the –CH3 proton signal into different separate signals. In addition, the left –CH2- can also be split into separate cis and trans signals.
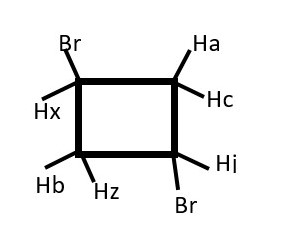
STEREOCHEMISTRY
However, the chemical environment and symmetry or orientation in space can cause hydrogens on the same carbon to couple each other’s signal as in NMR spectrum of compounds with two or more chiral centers.
However, a lower value for a doublet would mean the protons are farther away 4 bonds away or they are ENANTIOTOPIC protons or non-equivalent protons on the same carbon.
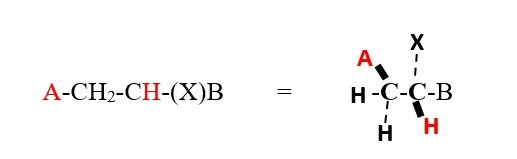
The proton on carbon attached to X and B is located on chiral center and is
-
Enantiotopic if A=H
-
DIASTEREOTOPIC if A is not H.
It interacts differently with the two protons near A because X and B will make the chemical environment of the two protons near A different. Thus we will get two doublets for protons near A with two different coupling constants for cis and trans. Protons near X and B will be TWO triplets with very close but slightly different chemical shifts at low temperatures.
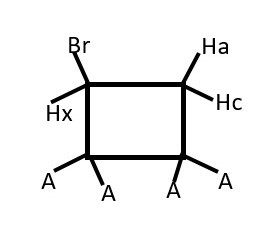
Rapid rotation at room temperatures will overlap the two triplets into just one triplet. Therefore, these difference in chemical shift are observed at lower temperatures.
Presence of Br or any group other substituent will make one phase of the structure become a different chemical environment from the other.
Thus, Hx (ENANTIOTOPIC) will interacts differently and separately with Ha and then separately with Hc. Therefore, we expect a doublet of doublets instead of the usual triplet from Hx.
Each Ha and Hc (DIASTEREOTOPIC) will also have separate but close doublets of different chemical shifts.
There will be a weak germinal coupling between Ha and Hc.
We should expect doublet of doublet of doublets from Ha and Hc that integrates to 2 non-equivalent protons.
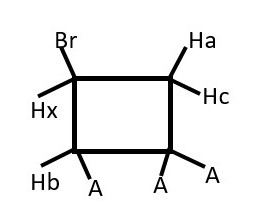
Hb will give a doublet but Hx will give doublet of doublet of doublets.
Identify homotopic protons if any
Identify enantiotopic protons if any
Identify diastereotopic protons if any
Predict signal peaks from Hb and Hx.
However, an alkene with cis and trans protons are fixed and the differences in the proton chemical shifts are clearly seen for cis and trans from their coupling constants.
EXAMPLE:
VICINAL Coupling of ALKENE protons.
CH3C(H)=C(H)CH3
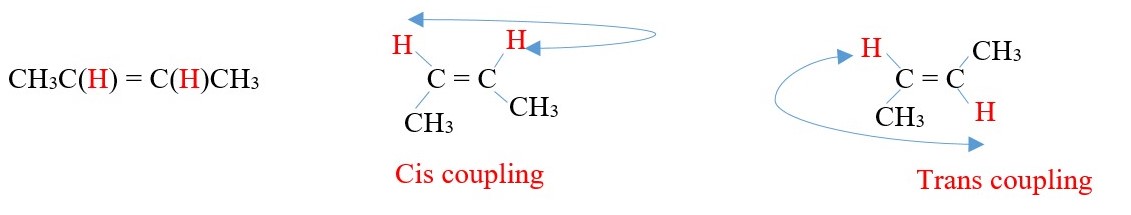
In vicinal coupling, the value of trans coupling constant is often larger than cis.
Sometimes germinal coupling will occur in alkenes.

FACTORS AFFECTING PROTON NMR
-
HYDROGEN BONDING affects peak shapes. A strong H-bonding can cause broad peaks to form for acidic protons, therefore, water impurity in sample will affect signal peak shapes usually showing as a very broad peak.
-
External field strength: a large field magnet can enhance peak intensity and give better resolution better than low field magnet but coupling constant values will remain the same.
e.g. a 400MHz instrument will give a much clearer resolved peaks in spectrum with the same amount of analyte than a 200MHz instrument but the coupling constants of the peaks and the chemical shifts will be the same.
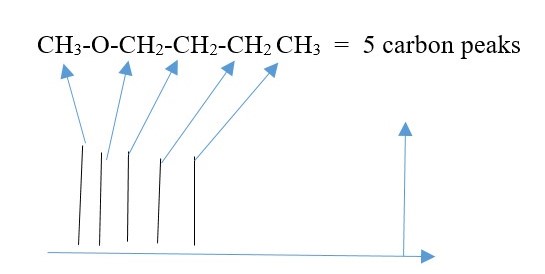
C-13 NMR
This is NMR due to Carbon -13 NUCLEI since C-12 is not NMR active because it does not have odd nuclei spin.
In this case, there is no coupling or splitting of signal peaks. Only individual carbons peaks are observed. Carbons in same chemical environment show as one but different if chemical environment is different. Again, shielded carbons give UPFIELD signal peaks, but de-shielded carbons give DOWNFIELD signal peaks.
Height of each peak is related to number of carbons causing it to form. Symmetry causes two or more carbons to show up as one peak. A signal peak from many carbons is higher than a peak from fewer carbons. Two symmetrical carbons giving of one peak will have greater height than peak from only one carbon.
EXAMPLE
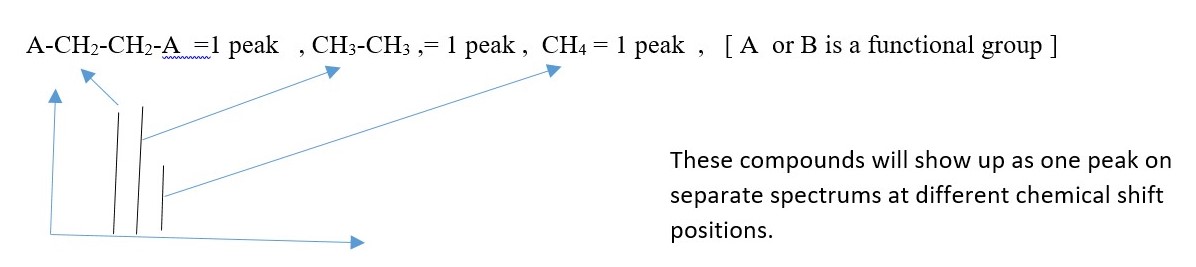
CH2B-CH2A = 2 peaks ,
CH3-CH2-CH3 = 2 peaks ,
CH3-BrC=CBr-CH3 = 2 peaks