ISOMERISM : STRUCTURE AND OPTICAL ACTIVITY
ISOMERISM:
Isomerism refers to the situation involving compounds of the same formula but differ in structure or spatial orientation. Therefore, we can have two main forms of isomerism, namely, structural isomerism and stereoisomerism.
STRUCTURAL ISOMERISM
This refers to compounds with the same formula but different structural formula. Knowledge and practice of how to write open chain structures, branching and functional groups of organic compounds is very important.
In this case it is very useful to know the Degree of Unsaturation of the given the formula of the organic compound.
DEGREE OF UNSATURATION
In any formula containing of organic compound containing C, H, O, N or halogen, you can calculate Degree of Unsaturation (DU) by ignoring the presence of oxygen and sulfur group elements and use the formula below.
Degree of Unsaturation = ([Hydrogens in parent straight chain alkane-Hydrogens in given formula] + [Nitrogen in given formula]- [Halogens in given formula]) / 2
Example: Find DU for a compound of formula C5H4NCl;
The given formula has five carbons, therefore n=5, and the parent straight chain alkane CnH2n+2 = C5H12 will have 12 Hydrogens
The given formula has 4H ,1N and 1 halogen (Cl) therefore DU= (12-4+1-1)/2 = 8/2 = 4
4 degrees of unsaturation means several possibilities for the structure of the compound and its structural isomers:
A) presence of 4 double bonds,
B). 3 double bonds and a ring.
C). two double bonds and 2 rings.
D). 1 double bond and 3 rings
E) 2 triple bonds.,
F)1 triple bond and 2 double bonds,
G)1 triple bond and 2 rings
H)1 triple bond and a ring and a double bond.
This should be very helpful in finding the structure of the compound and its isomers.
Example : Find degree of unsaturation for the formula of ethanol C2H6O
The Formula C2H6O means, n=2, therefore the parent straight chain alkane CnH2n+2, = C2H6, will have 6 hydrogens. Ignore oxygen atoms (oxygen and its group are not counted). Number of Nitrogen in given formula =0, Halogens in given formula=0. Therefore DU= (6-6+0-0)/2 = 0. This means the structure of the compound and its isomers will have no unsaturation (double bond or triple bond) or a ring. The compound and its isomers can have only single saturated bonds.
NOTE: Degree of Unsaturation (DU) only indicate the presence of unsaturation (double or triple bonds) or a ring (cyclic) in the structure of the compound or its isomers of the given formula. It does not tell you how many structural isomers that can exist for a given formula of a compound. Therefore DU=0 means the compound has no unsaturation and cannot have any isomer that is unsaturated or a ring (cyclic).
Example: Use DU to explain the if there will be unsaturation or ring isomers for a compound with formula C5H12.
This has DU= (12-12)/2 = 0. This means the compound is a saturated organic compound and can only have saturated structural isomers. Therefore, no unsaturated or CYCLIC (ring) isomer is possible in this case.
Stereoisomerism:
This refers the spatial arrangement of the structure of compounds. In organic compounds, this includes, Newman projections, E/Z isomerism (alkenes), Cis /Trans isomers (alkenes and alkanes), Enantiomers (alkanes) and Diastereomers (alkenes and alkanes) and more.
Newman projections:
This involves isomers of carbon compounds drawn such that one of the main carbons is in front and another one is behind. These carbons are represented as a dot (front carbon) in a circle (back carbon). The substituents on these carbons are drawn as seen in the actual structural formula. Different Newman projections can be drawn for the same compound because they have different energies or potential energies.
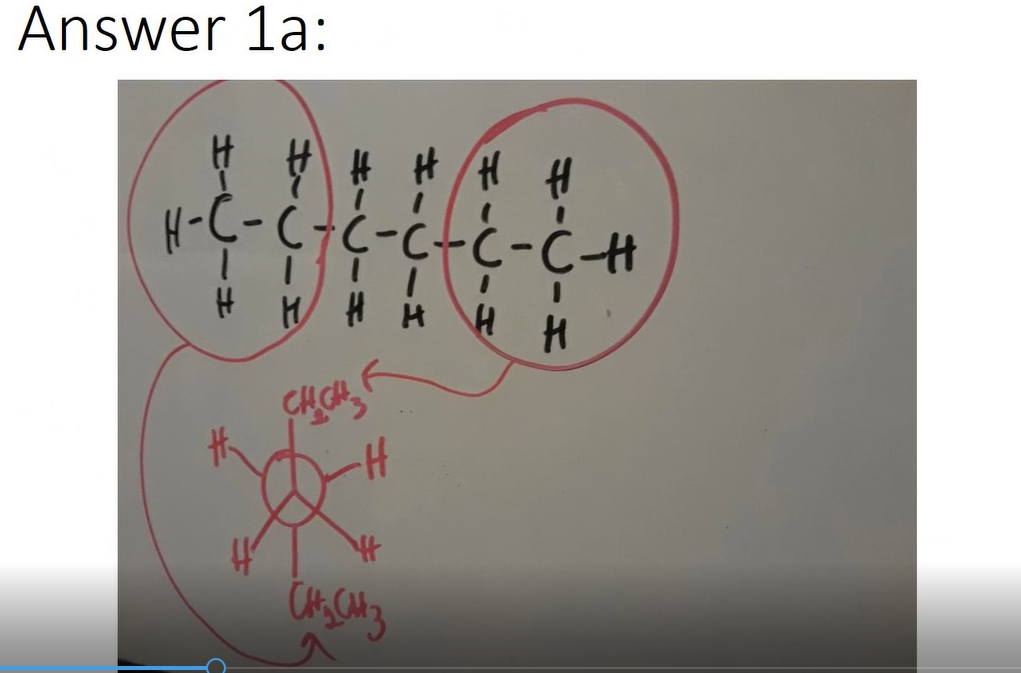
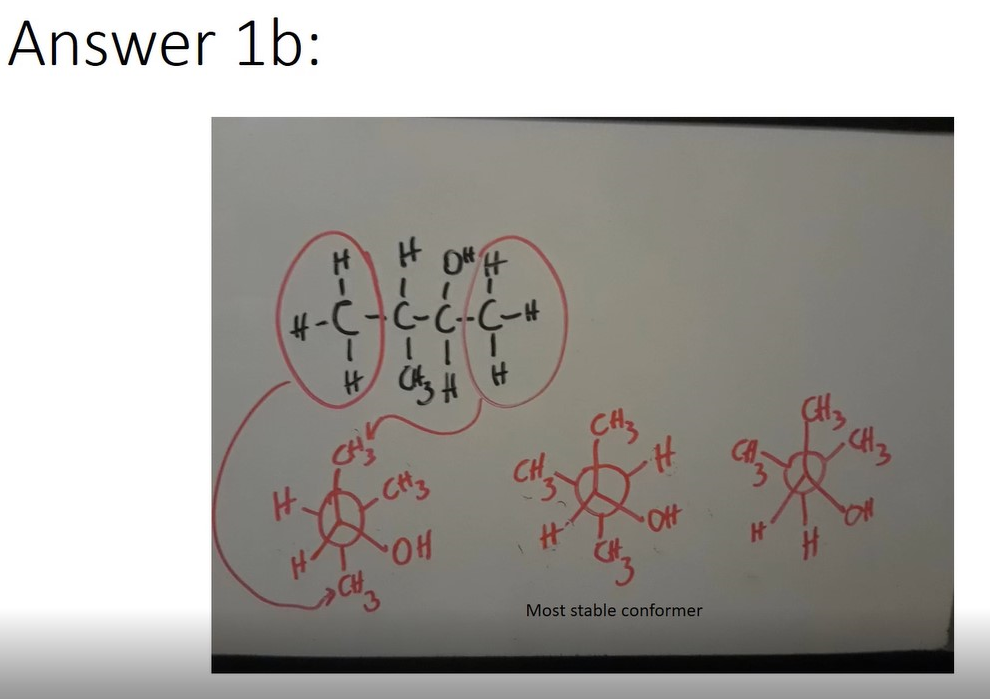
Example: Draw the most stable configuration
Questions:
- Determine the most stable Newman projection for a) Hexane and b) 3-methyl-2-butanol
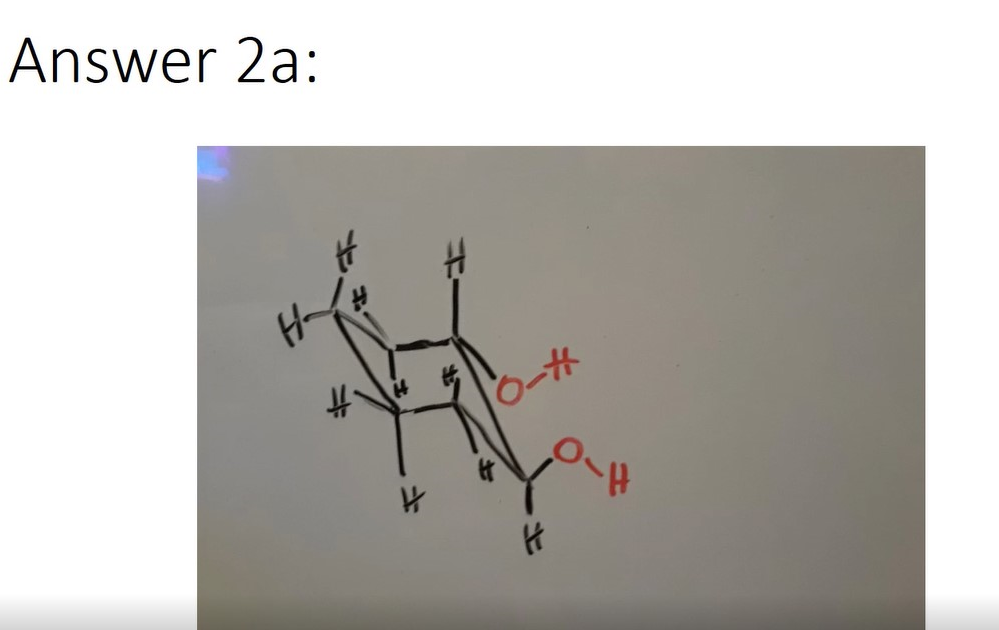
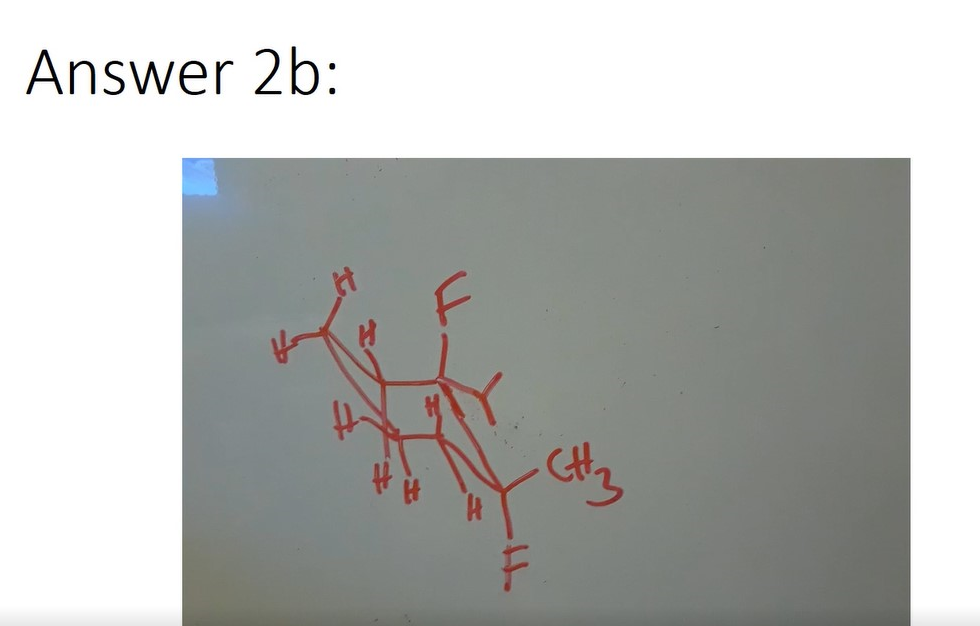
- Draw the most stable chair conformation for a) 1,2-cyclohexanediol and b) 1,2-difluoro-2-isopropyl-1-methylcyclohexane.
Alkene stereo-isomers
Alkene stereoisomers are diastereomers. This occur when the substituents at one phase of the double bond have the same spatial orientation or arrangement, but the other phase has different spatial orientation of the substituents. This includes.
Cis/trans; is used when the double bond has only two different substituents.
E/Z isomers; is used when double bond has two or more different substituents.
ALKANE STEREOISOMERS
Carbon in alkanes have 4 single bonds to substituents (atoms or groups). This gives them a 3-dimensional structure in space. Part of the structure directly pointing out towards the observer, the other part points away from the observer while the rest remain in the middle plane. This is like x,y,z spatial dimensions.
Chiral compounds and R/S Configuration:
Chiral compounds have 4 different substituents attached to the central carbon. The nature of the four substituents can cause the compound to rotate plane polarized light to the left or right. The four substituents allow characterization of chiral organic compounds in terms of R / S configuration.
Substituent priorities in chiral organic compounds:
Substituent distribution in R and S configuration depends on the molar mass of substituent directly attached to the chiral center. They are ranked 1= highest ,2,3,4 etc. Direction of molar mass distribution around chiral center determines the R or S configuration. Clockwise is R and counterclockwise is S.
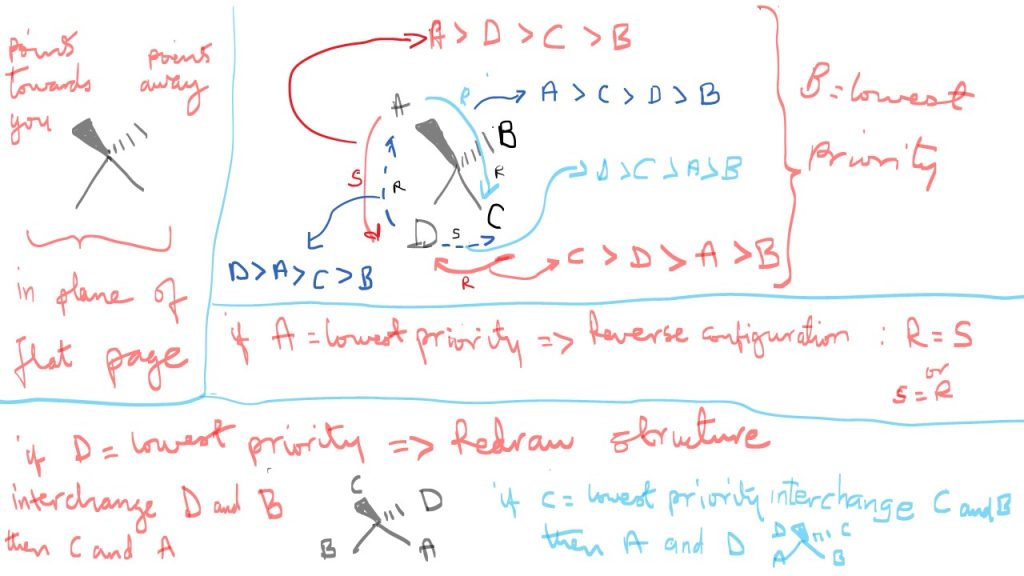
However, there are special substituents that are ranked by the oxidation state of the atom present in the substituent group. A higher oxidation state implies a higher priority. For example, carboxylic acid carbon is more oxidized and has a higher oxidation sate than the carbon in an ester. Also, the carbon in a triple bond is more oxidized than carbon in an alkene. In other words, a higher number of hydrogens lowers oxidation state, but a higher number of oxygen atoms increases the oxidation state of a substituent and hence its priority.
3-Dimensional arrangement of a molecule can be determined from spatial orientation of each chiral center and the whole molecule. Model kits are very useful in this topic.
Assigning R and S configuration:
Video illustration: How to understand 3-D arrangement of chiral center of enantiomers: Click here
Do not assign if your lowest priority is in the plane of the page unless you are using a model kit. You can always re-sketch the structure so that the lowest priority is pointing away from you. You can do this simply by interchanging any two substituents followed by the remaining two.
Enantiomers have opposite R and S configurations, and they have non-superimposable mirror images, or they are non-superimposable mirror image of each other. They have the same formula and mass. Molecules with single chiral centers can only have enantiomers, however if there is more than one center, we can have Diastereomers in addition to enantiomers. A diastereomers are two molecules that have one or more chiral centers of the same configuration (R/S) but opposite configuration at one chiral center. We can example of molecules with one chiral center, or two or more centers and cyclic chiral molecules.
Video: Enantiomers or diastereomers or same compounds
Video: Symmetry and meso compounds
Fisher projections reveal another way to draw 3-dimensional structure of organic molecules with two more chiral centers. This combines simple lines representations with bold wedges. This is very useful in writing open structures of bio-organic molecules such as glucose, sucrose, fructose and some aldose molecules.
Video : 3-Center chiral compounds and Fischer projections
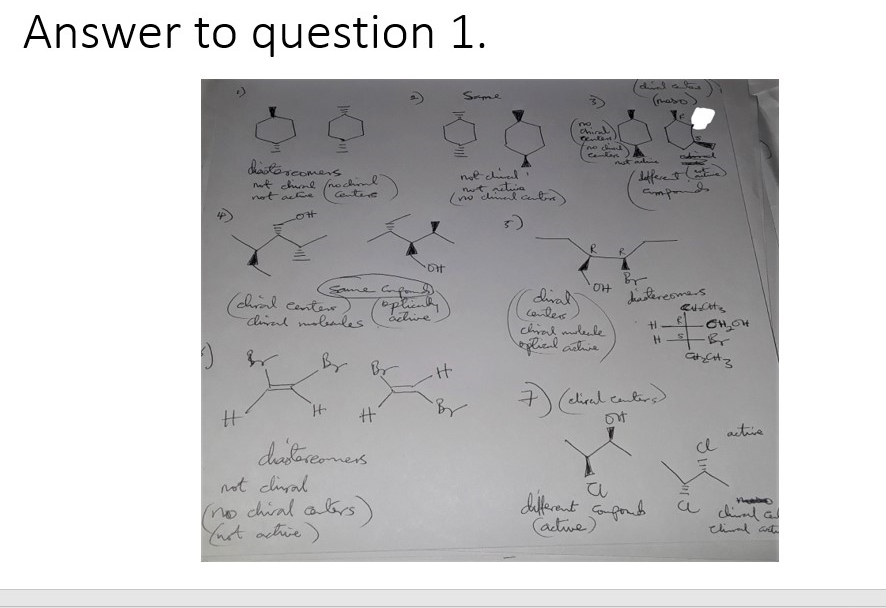
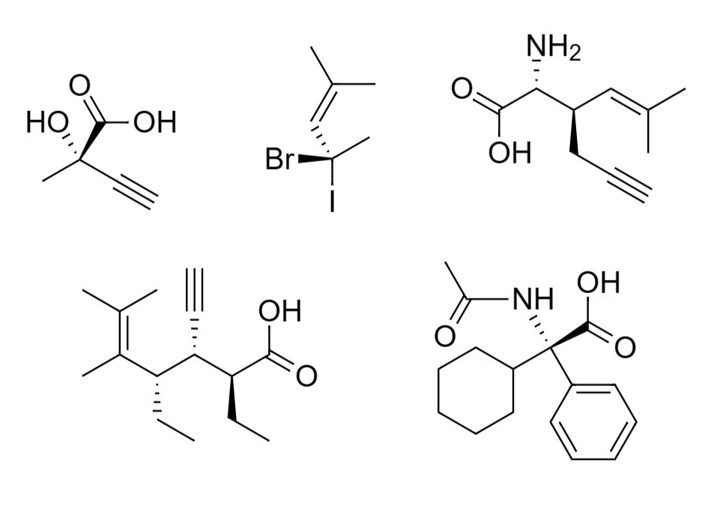
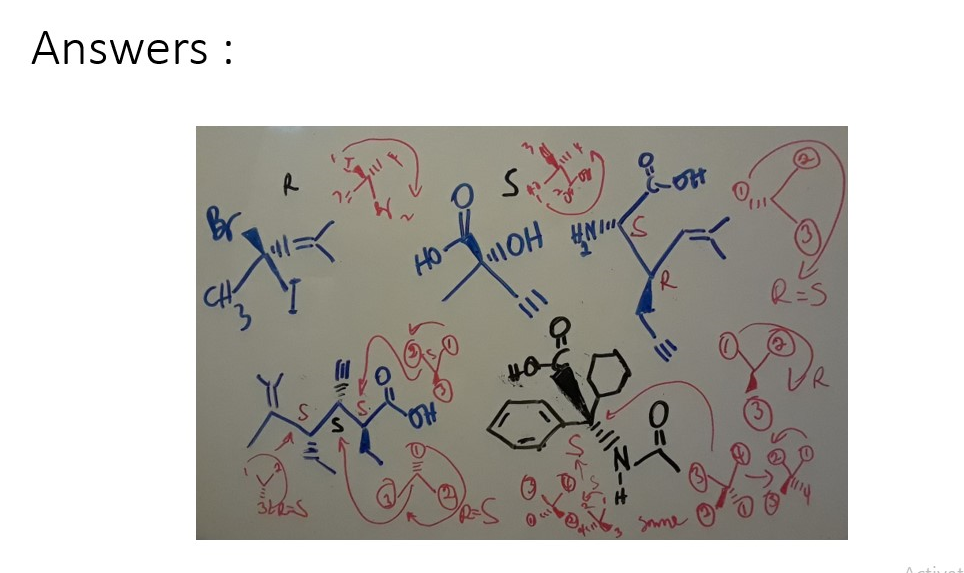
Question 1:
a) Identify the chiral centers in each molecule and determine the R and S configuration for each chiral center.
b) Determine which pair of molecules are enantiomers, diastereomers, same or different.
c) Which molecule is optically active and why?
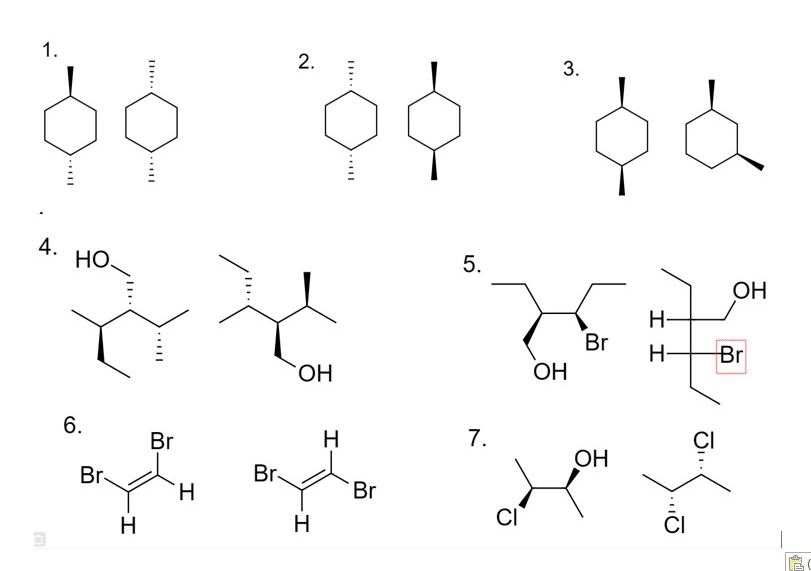
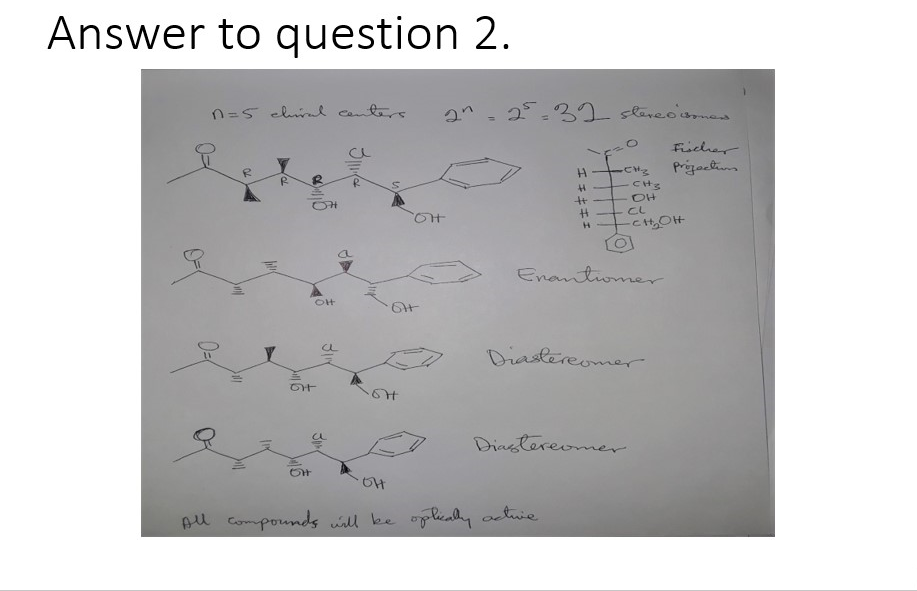
Question 2:
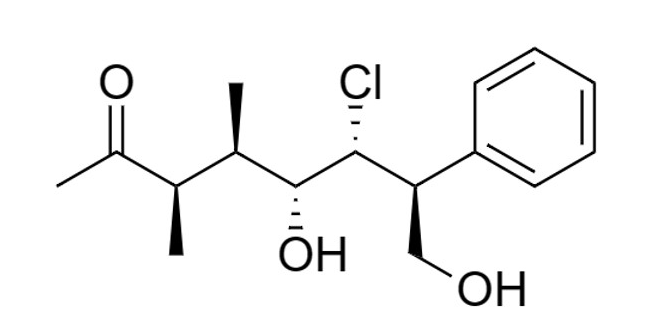
a) Identify the chiral centers in this molecule and determine the R and S configuration for each chiral center in the molecule above.
b) How many stereoisomers can be obtained from this molecule
c) Draw the enantiomer and two diastereomers of the molecule.
d) Draw the Fischer projection of the molecule.
Question:
Identify the chiral centers and determine the R/S configuration for the structures below.
HOW TO IDENTIFY COMPOUNDS: THE SAME OR NOT IDENTICAL OR ENANTIOMERS OR DIASTEROMERS
Compounds are the SAME: If the number of atoms is the same, the atoms are the same, the bonds are the same kind and number, and the stereochemistry is the same.
AXIAL CHIRALITY
Axial chirality is the chirality that a compound possesses due to the shape of its structure but not due to the presence of any chiral center. Compounds that show axial chirality include allene, spirocyclic compounds and Atropsisomers.
A) Allene and odd numbered cumulenes
These have terminal carbons that are twist out of phase making them non-planar. If the terminal carbons have different substituents, the allene will be chiral. R and S configuration is determined by the two substituents on each terminal carbon i.e. [ high(h) priority or low(L) priority]. Therefore, these compounds have chiral axis and are optically active. The priorities of substituents are assigned in the same way as in any other chiral compound. These compounds can be achiral if the substituents are the same on one carbon; [that is A=B or A’=B’] since this will create a plane of symmetry in the molecule.
B) Atropsiomers
These have a restriction of free rotation around a single bond caused by certain substituents. This creates a chiral axis in the structure. The chiral axis that is used to determine the R and S configuration is located on four carbons bearing the substituents that restrict free rotation around single bonds. Chiral atropisomers are optically active. The substituents priorities are determined as in any other chiral compound. The substituents should be large enough to restrict free rotation of the rings at room temperature or below. In this case A and B can be the same substituent but both must not be hydrogen at the same time to maintain chirality. The substituents priorities [high(h) or low(L)], are determined as in any other chiral compound. Chirality is maintained even if two substituents are the same. i.e. [(A and A’), (B and B’), (A and B), (A’ and B’), (A and B’) or (A’ and B).]
C) Spirocyclic compounds
These have a spiral twisted part of a ring in a spatial orientation out of phase with the other part. The spiral twist in the structure of compounds can give them chirality. In this example, the R and S configuration is determined by the substituent priorities (High(h) or Low(L)) on the four carbon atoms next to the spiral twist center. However, unlike in ALLENES and in ATROPISOMERS, the Spirocyclic compounds, have A and A’ (as well as B and B’) assigned diagonally from each other to get the chiral axis.
The chiral axis originates from the 3-DIMENSIONAL representation of the structure of the compound.
a) parts of the structure in the flat plane (simple lines).
b) part of the structure going into the plane of the page pointing away from observer(dashes).
c) part of the structure that is coming out of the plane of the page and pointing towards observer (solid bold).
Try and use the above procedure to identify the R/S configuration for BINAP and its derivatives.
Molecular Orbital Theory
Free radical reactions
Electrocyclic reactions:


