Balancing chemical equations
Balancing chemical equations:
Important to remember that we only WRITE COEFFICIENT NUMBERS; whole numbers written in front of an alphabet [ Also known as MOLES] to balance a chemical equation.
E.g. A is the same as 1A that is 1 x A = A, therefore A has coefficient of 1 , 2A coefficient of 2 , 3B has coefficient of 3.
We do not use SUBSCRIPTS OR SUPERSCRIPTS to balance a chemical equation.
-
A ____> A2
-
We cannot write A2 _____> A2 instead we write 2A ______> A2 ………[balanced]
Let balance this; A + B _______> A2B5
List the items or elements and count on each side of the equation.
| A | B | |
| Left side | 1 | 1 |
| Right side | 2 | 5 |
Now multiply through by using coefficients to make left side equal to the right side of the equation.
| A | B | |
| Left side | 1x2 | 1x5 |
| Right side | 2 | 5 |
Re-write equation.
2A + 5B ______> A2B5 …………[balanced equation]
SPLIT METHOD
An element that appears in two or more different compounds at the same side of an equation must be counted separately for each compound instead of the sum.
E.g. In the illustration below, Element B appearance in two compounds at the left side of this equation below can cause the balancing of the equation to be complicated but it is made easier by splitting the number of B into two (5 and 3) at the left side in the list instead of writing the sum 8.
Let balance this; A2B5 + B3C2 ____> AC3 + B
List the items or elements and count on each side of the equation.
| A | B | C | |
| Left side | 2 | 5+3 | 2 |
| Right side | 1 | 1 | 3 |
Now multiply through by using coefficients to make items on left side equal to the right side
| A | B | C | |
| Left side | 2 | 5 + 3x3 (second step) =14 | 2x3 |
| Right side | 1x2 (first step) | 1x14 (third step) | 3x2 |
A2B5 + 3B3C2 ____> 2AC3 + 14B ……….[balanced equation]
GROUP METHOD
Polyatomic ions appear as group in a compound, but these can be listed as one entity for each compound if they appear the same in both sides of the equation [left (reactant) and right (product) side].
In this illustration, below the polyatomic groups are YZ and CHD. These are counted as single entities since they appear the same on both sides of the equation. This makes it easier to balance the equation given.
E.g. Let’s balance this; A(YZ)2 + BCHD ____> A(CHD)2 + BYZ ……..[not balanced]
List the items or elements and count on each side.
| A | B | CHD | YZ | |
| Left side | 1 | 1 | 1 | 2 |
| Right side | 1 | 1 | 2 | 1 |
Now multiply through by using coefficients to make left side equal to the right side of the equation.
| A | B | CHD | YZ | |
| Left side | 1 | 1x 2 (second step) | 1x 2 | 2 |
| Right side | 1 | 1x 2 (first step) | 2 | 1x 2 |
A(YZ)2 + 2BCHD ____> A(CHD)2 + 2BYZ………….[balanced equation]
EXAMPLES
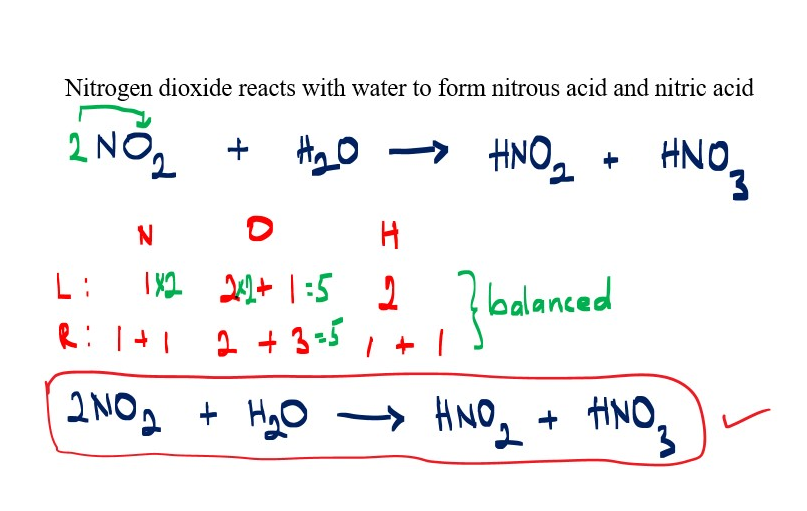
Reaction of nitrogen dioxide with water
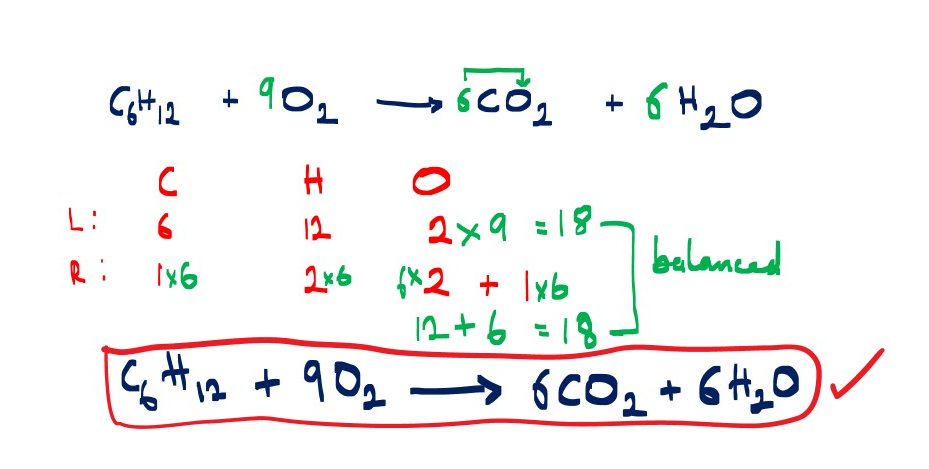
Complete combustion of hexene in oxygen gas
Reaction of Sodium hydroxide with sulfuric acid
NaCl + H2SO4
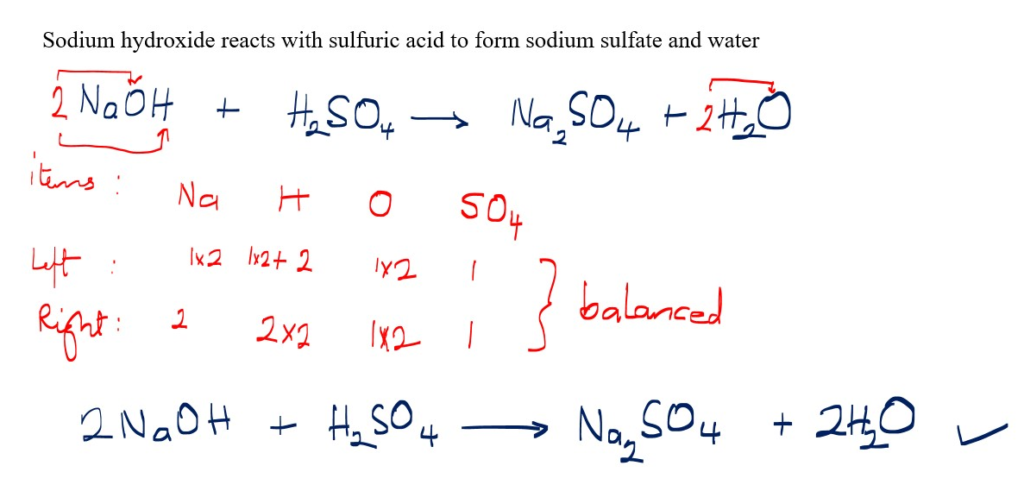
How to balance more difficult equations
BALANCING IONIC EQUATIONS
This is a follow up of balancing equations;
Balance equations without ions are MOLECULAR EQUATIONS.
Ions of elements that form soluble ionic compounds; group 1 and 2 elements, e.g., K+, Na+, and all group 1 metal compounds etc.
Polyatomic ions that form soluble ionic compounds; e.g. NO3–, NH4+, ClO4–, ClO3–
Ions that form insoluble compounds or precipitates; e.g. OH–, SO3-2, CO3-2, S-2, PO4-2-
Presence of any soluble ion makes the whole compound soluble even if an insoluble ion is present.
Only Soluble compounds [aka AQUEOUS= (aq)] can be separated into constituent ions. Every other state cannot be separated; e.g. Solid (s), liquid (l) or gas (g).
Use COEFFICIENT NUMBERS to show the number of ions or balance equations. E.g. K3PO4 is; 3 K+ ions in contact with a PO4-3 ion. The Al(NO3)3 is one AI+3 ion in contact with 3 NO3– ions.
Spectator ions are the same on both left and right side of equation and cancel out to give overall ionic equation.
You can determine if there is a chemical reaction by checking if anything other than aqueous if formed [that is solid (insoluble) =precipitate(s), liquid(l) or gas(g) is formed.]
Example 1
Write a balanced the ionic reaction between sodium hydroxide and hydrochloric acid to form water.
NaOH + HCI _______> NaCI + H2O … [balanced already]
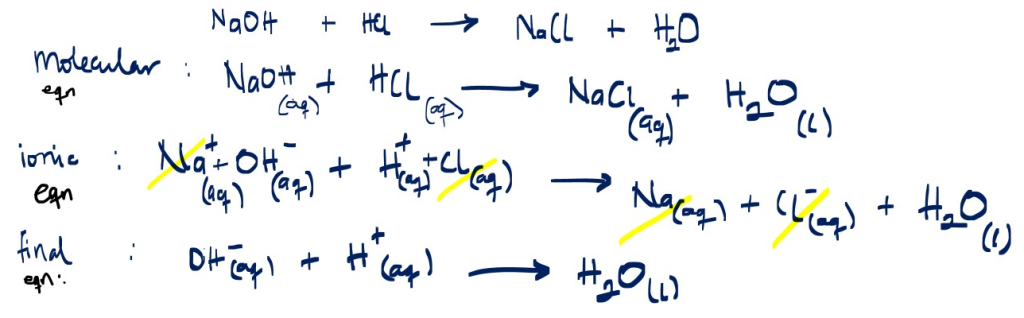
2. Write the overall balanced ionic equation for reaction below;
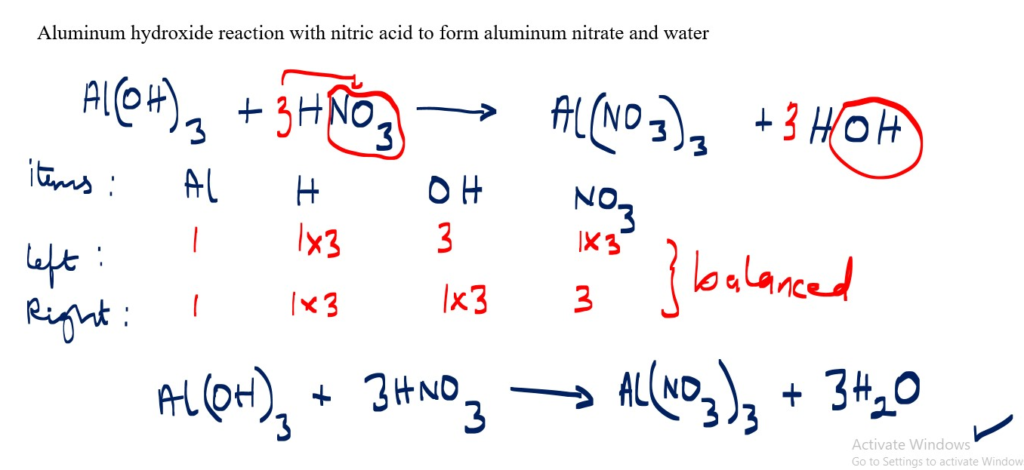
Aluminum hydroxide reaction with nitric acid to form aluminum nitrate and water
Al(OH)3 + HNO3 _______> AI(NO3)3 + H2O [not balanced]
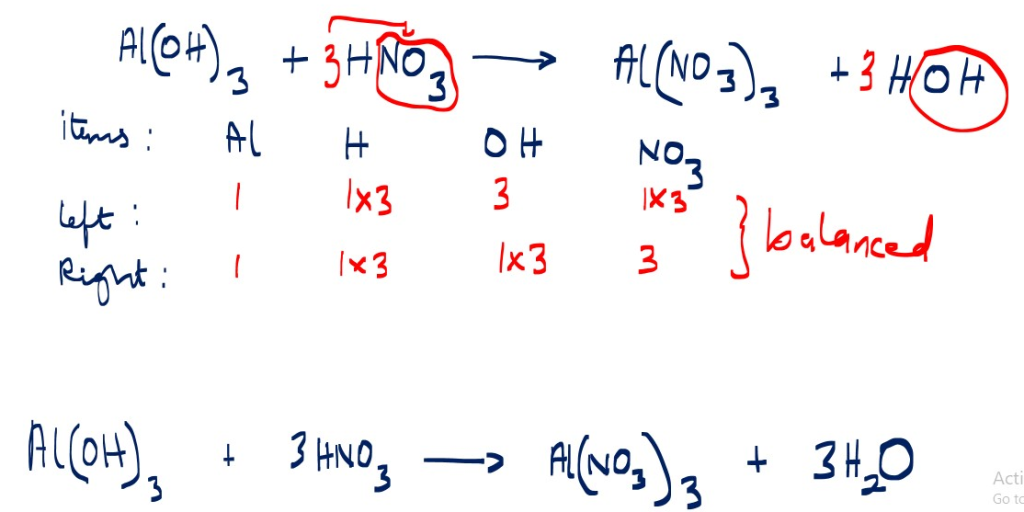
Ionic equation
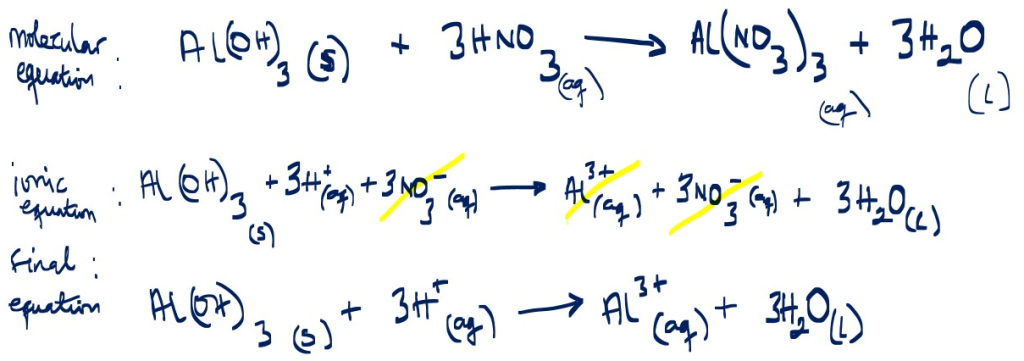
Example 3
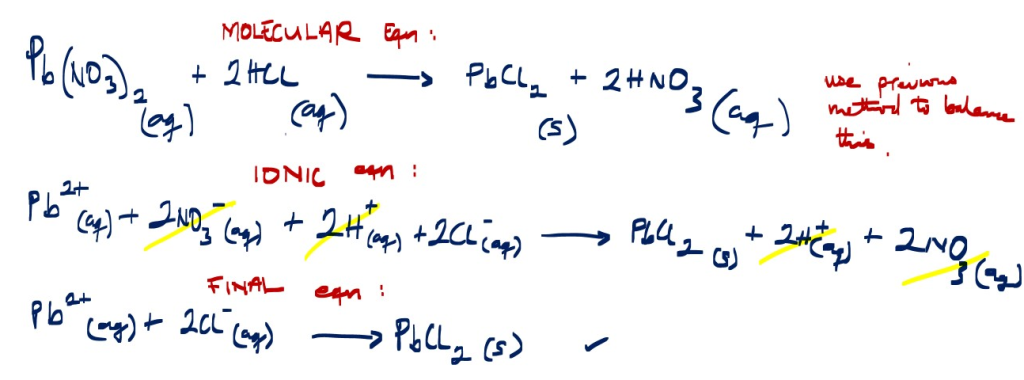
Write a balanced reaction between lead (ll)nitrate and HCl
Pb(NO3)2 + HCl
Reaction between lead(ll)nitrate and HCl forms lead(ll)chloride and nitric acid
Pb(NO3)2 + HCl ________> PbCl2 + HNO3 …… [unbalanced equation]
P.S. use the previous above method of (SPLIT AND GROUP) to balance the molecular equation.