ELECTROCYCLIC AND CYCLOADDITIONS REACTIONS
Electrocyclic reactions:
Electrocyclic reactions are intramolecular reversible reactions involving a single starting compound in which electrons are simultaneously exchanged between double or triple bonds to form a new sigma bond and a cyclic structure. They are addition reactions because the sum total of the number of atoms in starting materials equals the number of atoms in the product.
Electrocyclic reactions are neither homolytic nor heterolytic reactions.
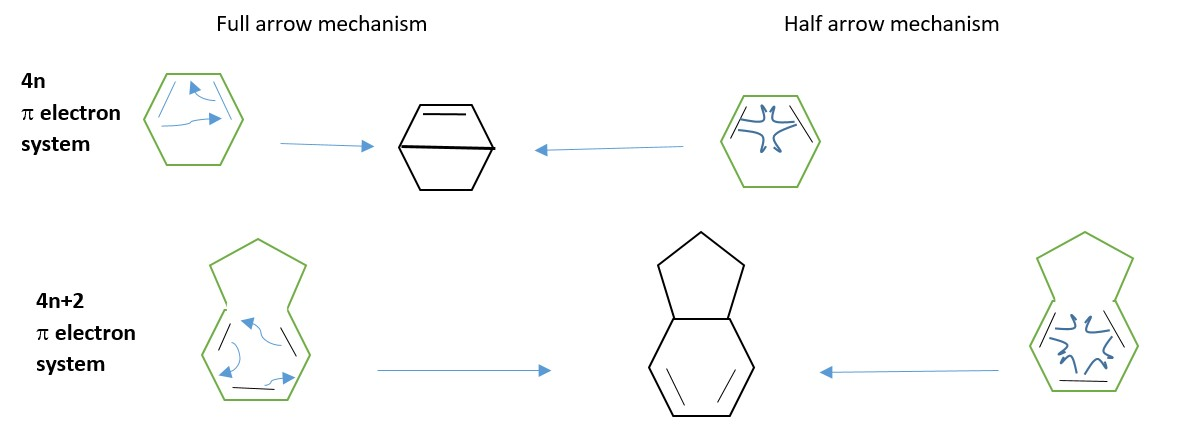
The reaction is usually illustrated with the full arrow mechanism whereby each full arrow represents a pair of electrons. However, it is sometimes shown as half arrow mechanism whereby half arrows representing single electrons within their π-bonds to be shared to form a new sigma bonds.
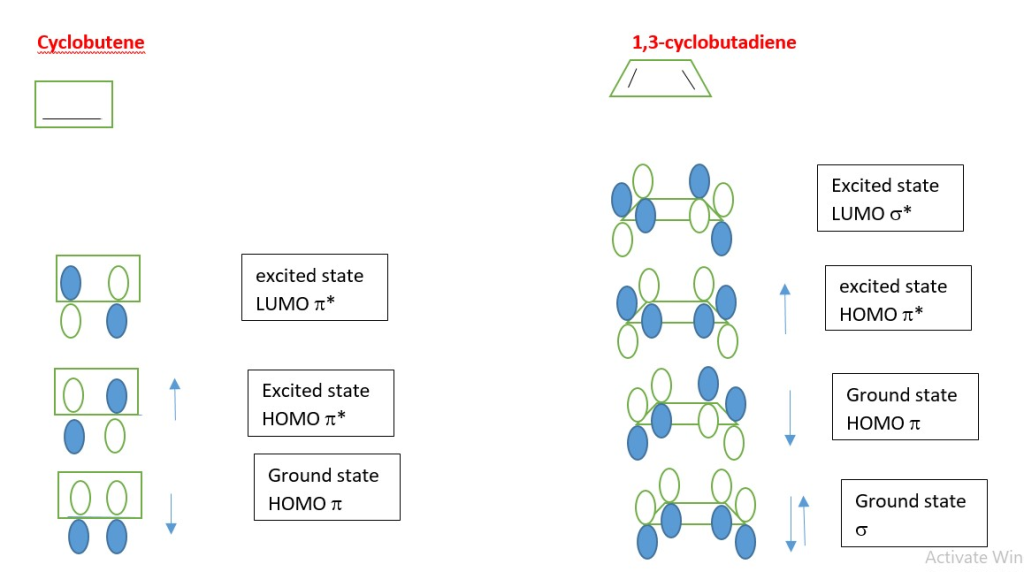
π-electron systems can be
- 4n where n=1,2, 3… example 1,3-butadiene
- 4n+2 for n=0,1,2,3……example ethene, benzene, 1,3,5 hexatriene
Electrocyclic reaction will convert one π-system to another.
EXAMPLE :
MOECULAR ORBITAL THEORY and ELECTRON SYSTEM
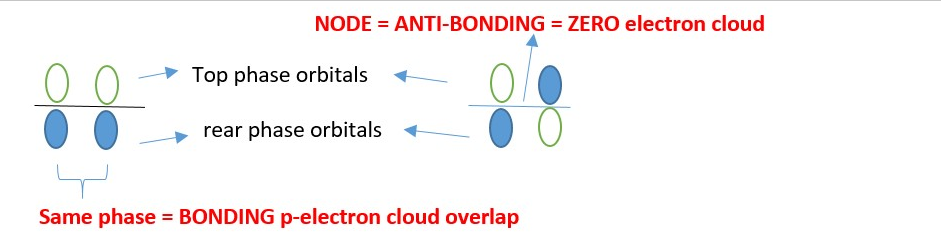
A p-system is made of p orbitals overlap in different phases. When the same phase lobe overlap bonding occurs but different phases of the p-orbital lobes adjacent on same level forms a NODE and becomes ANTI-BONDING (*) = Zero or no bonding electron cloud occur in that overlap area.
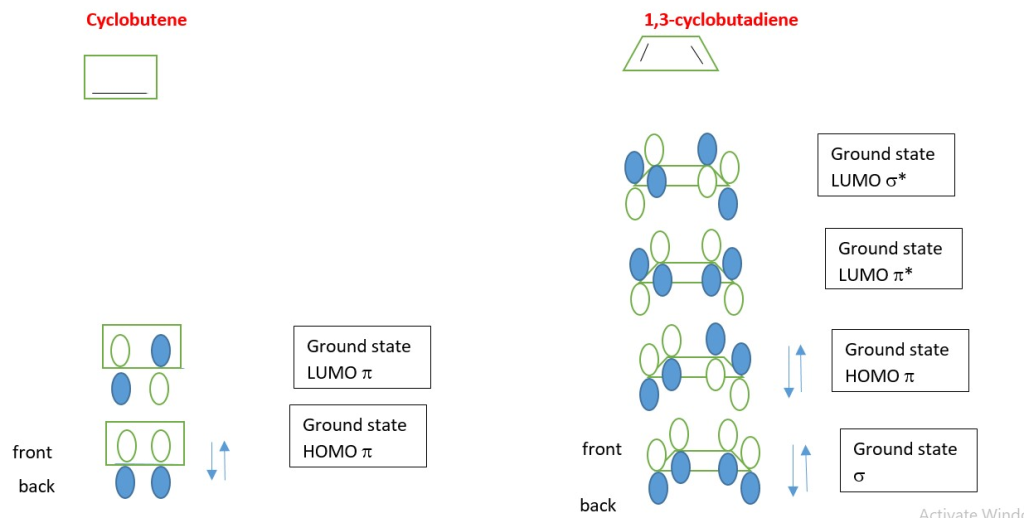
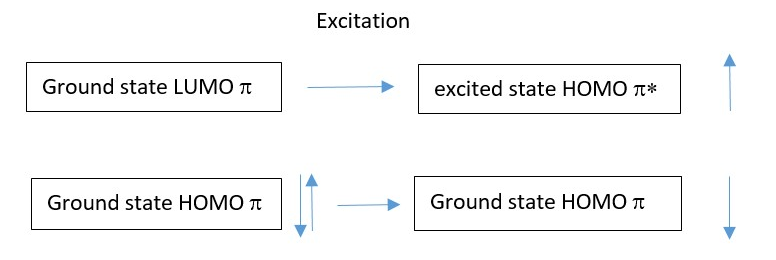
In the excited state the electron in the HOMO absorbs light energy of the right wavelength and the electron is promoted to the LUMO which becomes the new HOMO excited state.
MO THEORY, BONDING and Chemical Reaction conditions:
A Chemical bond occurs when a p-electron cloud overlaps with an empty p-orbital of the same phase.
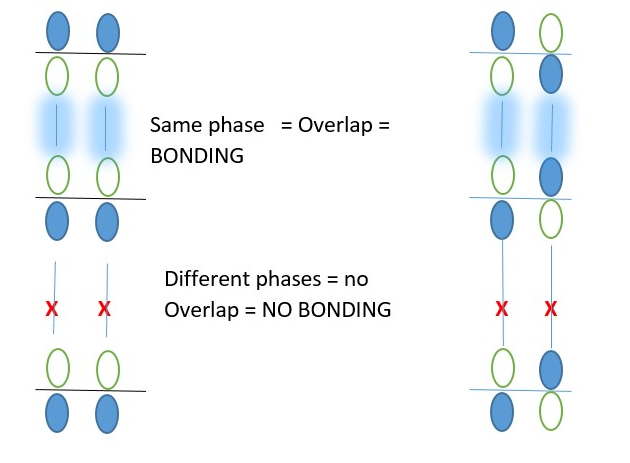
The front lobes of the orbitals of a p-system should interact with the front lobes of the another p-system to prove if an overlap will give bonding or non-bonding interactions. In other words, frontier orbital overlap shows bonding and non-bonding interactions.
If the back inner end lobes of a p-system are used, they should interact with the back inner end lobes of another p-system to prove that it is bonding or non-bonding interactions. E.g. Diels Alder reaction.
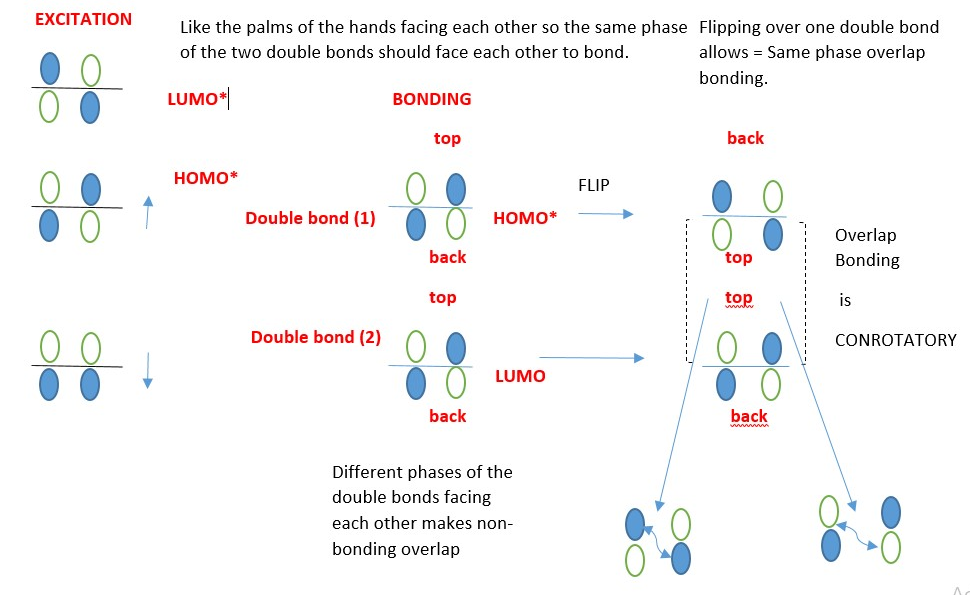
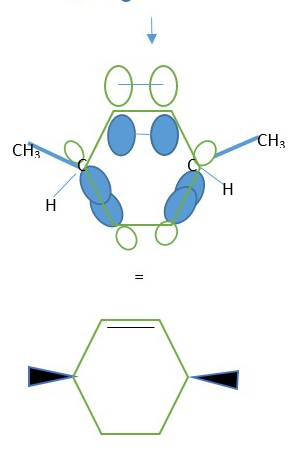
Conrotatory motion: Both p-orbitals of the two =C-R carbons move clockwise or Anti-clockwise directions to form the sigma bond to close the ring.
A Chemical bond occurs when a p-electron cloud overlaps with an empty p-orbital of the same phase.
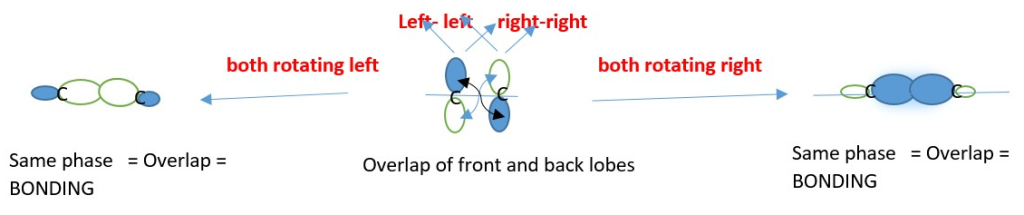
These rotations are promoted by light or heat.
This directly relates to the way =C-R frontier carbons CAN BE ROTATED so their p-orbitals can overlap in the same phase to form the ring closing sigma bond and establish stereochemistry in the products. The two carbons that closes the ring rotates from horizontal [ p-bond] to vertical [sigma bond] and vice versa for ring opening.
PRACTICE: Use two pencils.
Top and tip represents the two different groups attached to ending =C-R frontier carbons that form the sigma bonds from π-bonds.
The two pencils orient horizontally when starting with alkene =C-R but moves into a perpendicular vertical position to close ring during cyclization.
Conrotatory : both pencils move in same direction to form ring.
Disrotatory: one group =C-R moves opposite in direction to reach vertical position, [that is one moves clockwise and the other moves Anti-clockwise].
Determine the STEREOCHEMISTRY: Assign Dash wedges to groups is pointing downwards but Bold wedges to groups pointing upwards.
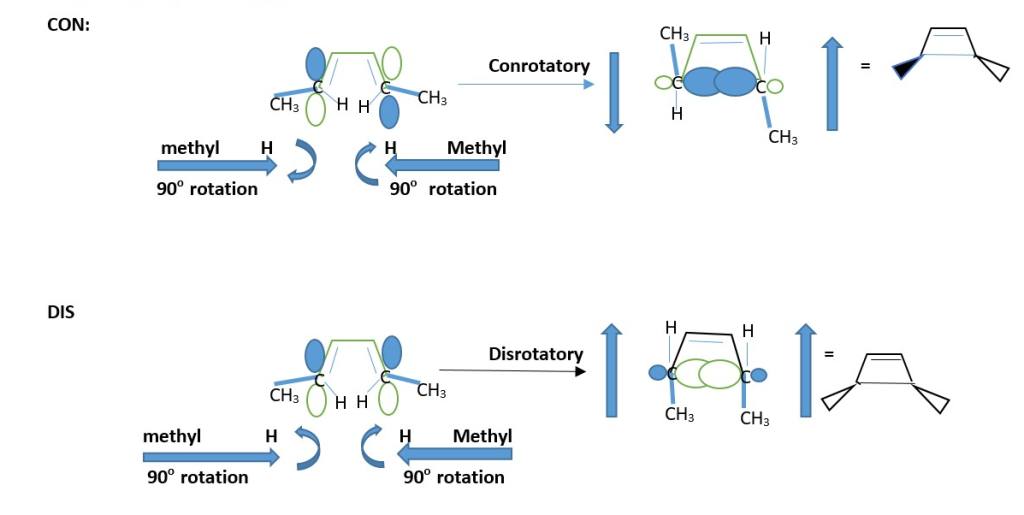
Conrotatory cyclization is promoted by light in 4n + 2 π -systems but promoted by heat in 4n π-system.
Disrotatory cyclization is promoted by heat in 4n + 2 π-system but promoted by light in 4n π- system.
MO THEORY, BONDING and Reaction conditions:
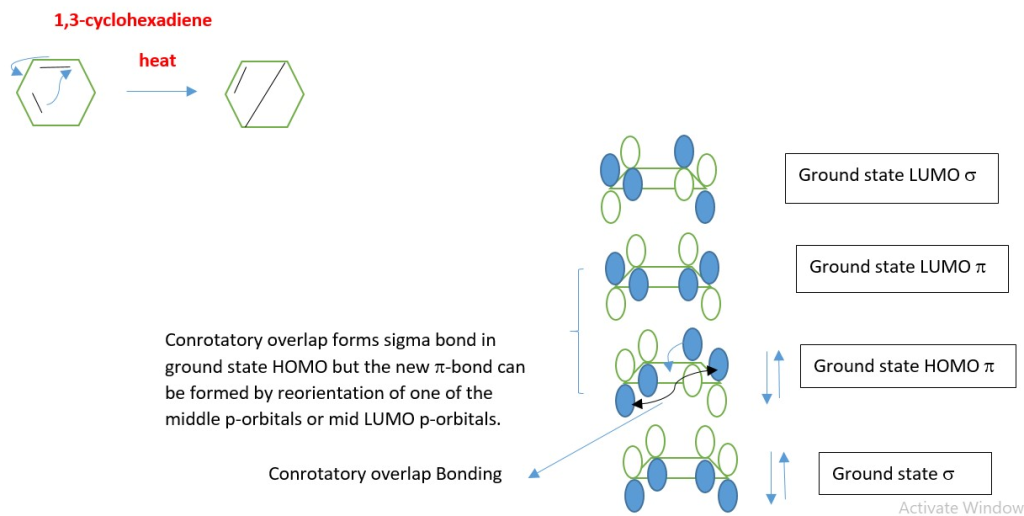
1. HEAT/THERMAL ELECTROCYCLIC REACTIONS
If heat is used for electrocyclic reaction, electrons in the ground state HOMO π-electron system interact with the ground state LUMO to form a new sigma bond and new π-bonds.
[4n] π-electron system
[4n+2] π-electron system
EXAMPLE : Conversion of 1,3,5-hexatriene to 1,3-cyclohexadiene .


LIGHT/PHOTO ELECTROCYCLIC REACTION
If light is applied, electrons in one p-electron system absorb energy and are excited from the ground state HOMO into the next higher level HOMO [first excited state triplet HOMO*].
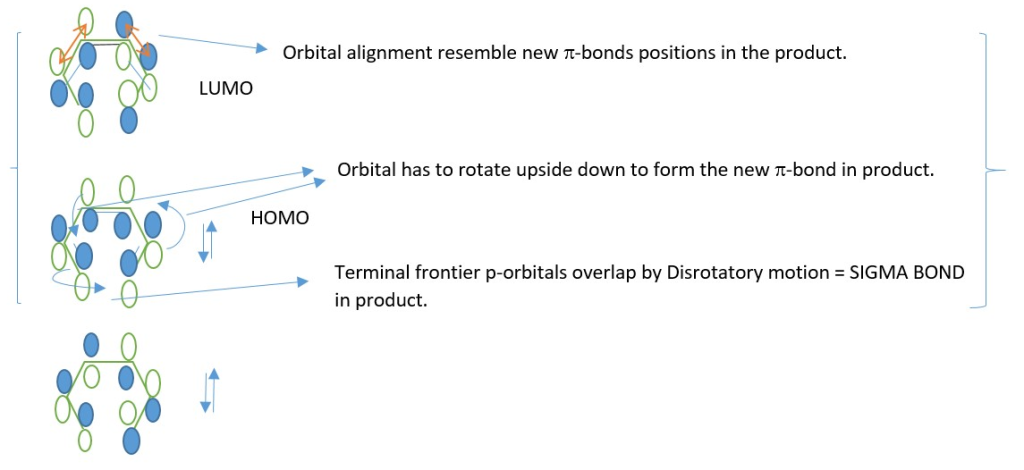
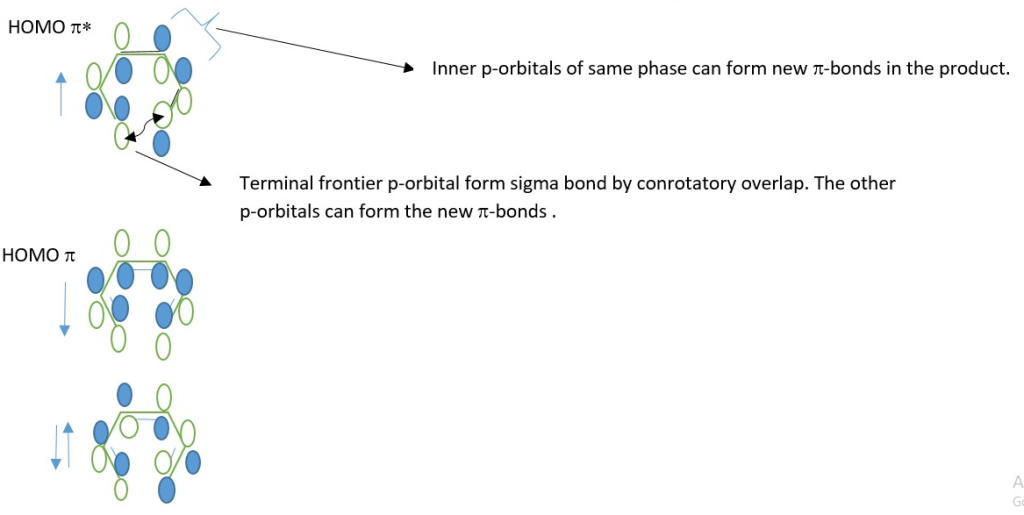
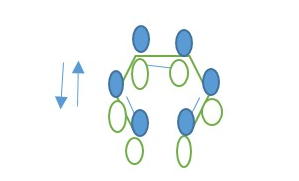
In a 4n π-electron system, New sigma bond form by Disrotatory overlap of terminal p orbitals and new p-bonds form from the same HOMO*.
In a 4n+2 π-electron system, New sigma bond form by Conrotatory overlap of terminal p orbitals and new π-bonds form from the same HOMO*.
[4n] π-electron system
This occur through interaction of Excited state HOMO* and a ground state LUMO.
π* = excited state π molecular orbital.
Example :
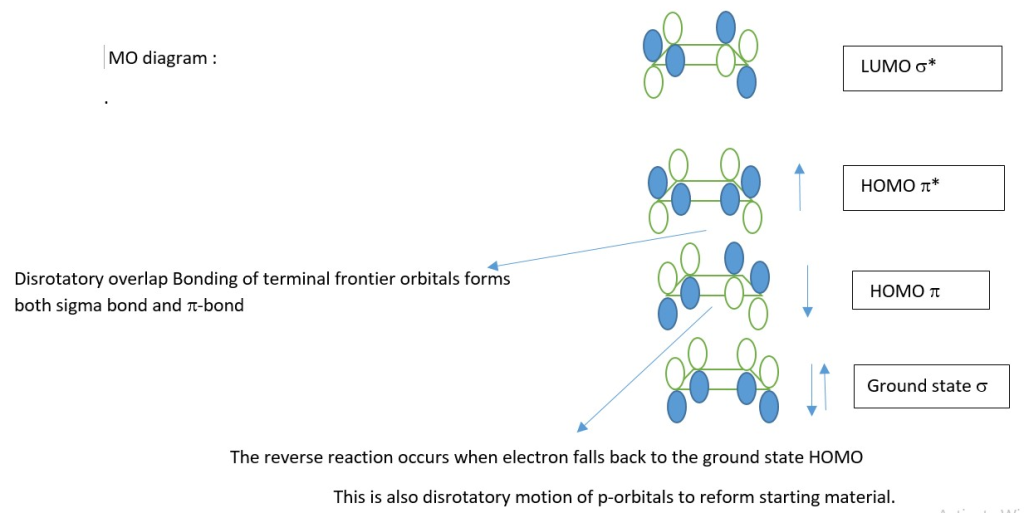
[4n+2] π-electron system
Example : Photo conversion of 1,3,5-hexatriene to 1,3-cyclohexadiene
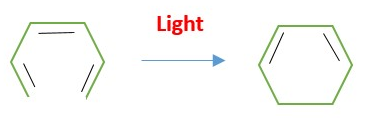
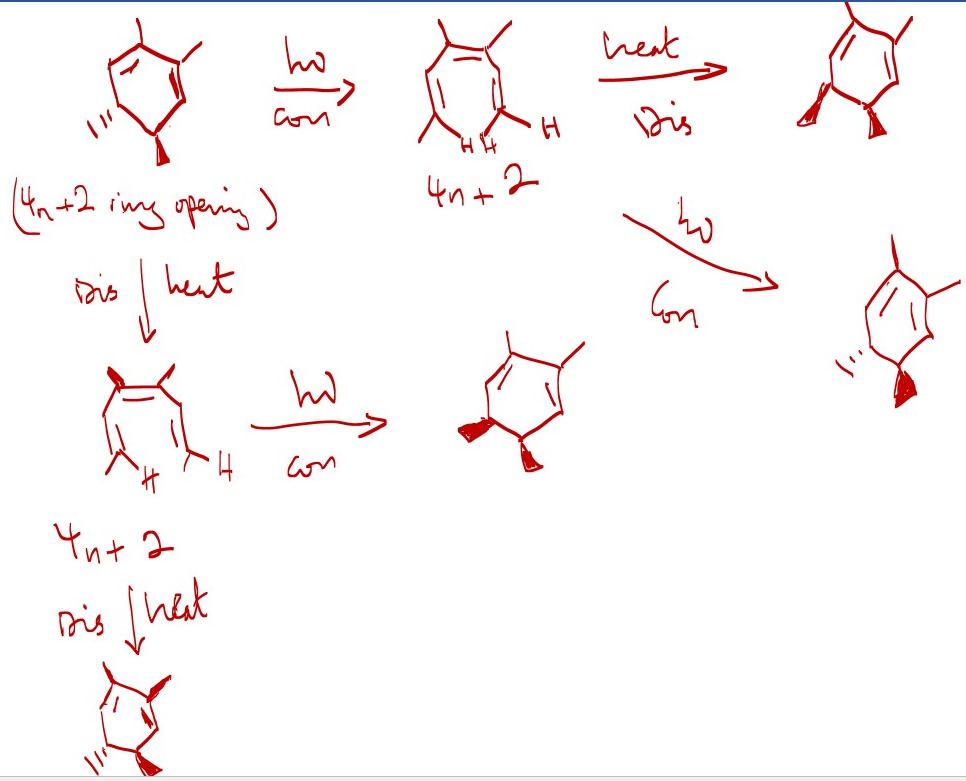
Electrocyclic reactions [4n or 4n+2 systems] are reversible in the same reaction condition [heat or light]. Mechanism [Conrotatory or Disrotatory] is the same for both forward and reverse reactions.
- Cyclic ring formed by heat can be reversed to the starting alkene material by applying heat.
- Cyclic ring formed by light is reversed to starting compound by applying light.
- However, interrupting or changing the reactions conditions [ applying heat and light and vice versa] changes the stereochemistry of product. Example shown below.
CYCLOADDITION REACTIONS:
Cycloaddition reactions are electrocyclic reactions that consist of two starting materials.
Dienophile has one double bond or triple bond. A Diene has two conjugated Syn – Cis double bonds.
Naming of cycloaddition is simply given by the number of electrons used in the reaction.
Cycloaddition reactions are inter-molecular whereby the groups exist in two separate molecules or intramolecular whereby the groups occur in the same molecule. The two species to approach each other in the correct position or orientation to form the bond.
Cycloaddition reactions conditions; THERMAL reactions occur with all orbitals in ground state only. PHOTOCHEMICAL reactions includes orbitals in excited state since they occur with excitation of electrons into orbitals in higher energy state. It turns a ground state LUMO into an excited state HOMO.
[2+2] cycloaddition
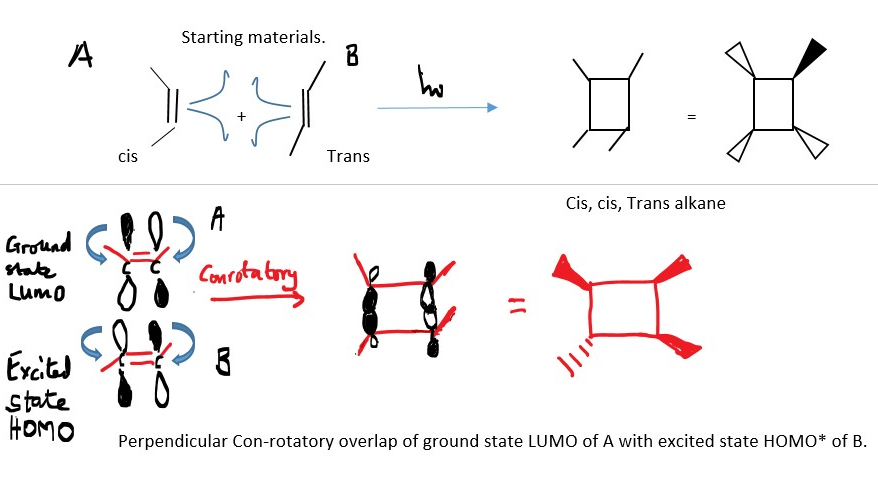
2+2 cycloaddition is not thermal because ground state HOMO of one reactant does not overlap in the same phase with the ground state LUMO p-orbitals of another p-system but the 2+2 Cycloaddition reaction is mainly photochemical reaction because the excite state HOMO frontier orbitals OVERLAP in the same phase with the ground state LUMO of another p-system in a Conrotatory motion to form sigma bond.
EXAMPLE:
Explain why the reaction below is photochemical and not thermal.
ethene + ethene = cyclobutane
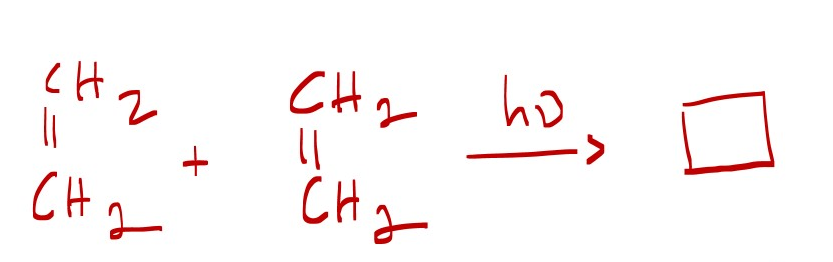
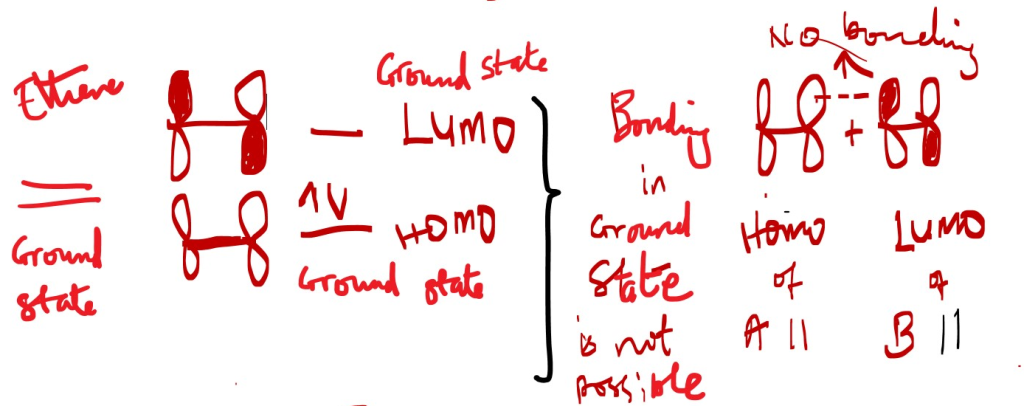
A Chemical bond occurs between the excited state HOMO* of one molecule and a ground state LUMO of another molecule with an empty p-orbital of the same phase.
The front lobes of the orbitals of a p-system should interact with the front lobes of the another p-sytem.
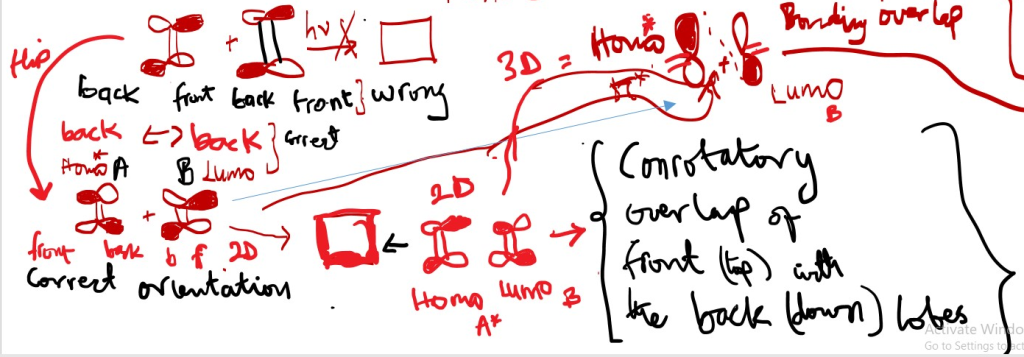
The structures are 3D ; the double bonds are flat in the plane of the page but the p-orbitals are actually perpendicular in that the right side [front side] is pointing towards the observer while the left side [back end] points away in opposite direction.
Before forming a bond flip one of the compounds so the orbitals can form bonding overlaps in 2D, however to determine if bonding occur by con or dis-rotatory, use 3D model of structure where the right side of compound orbitals points towards the observer while the left side p-orbitals points away. Overlap of the p-orbitals in the 3D-model is the proof of CON or DISROTATORY.
A Chemical bond occurs when a p-electron cloud overlaps with an empty p-orbital of the same phase.
EXAMPLE :
The reaction between a triple bond and a double bond is an example of cycloaddition reaction. [https://www.nature.com/articles/s41467-020-16283-9] .
Triple bond has two p-bonds and only one containing two p-electrons is used at a time. The other p-bond can also be used for another reaction. The stereochemistry of the alkene product is determined by the stereochemistry of the alkene starting material.
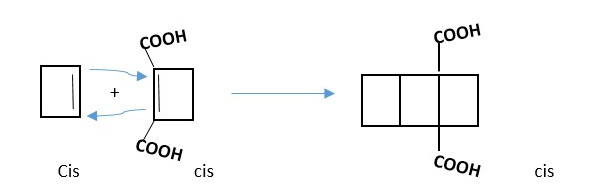
TRY: Write a 2 + 2 cycloaddition reaction between two 2-butyne molecules.
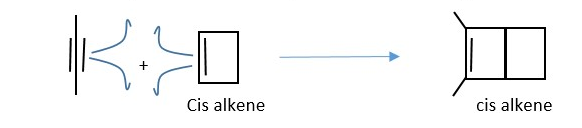
TRY : Reaction of two trans 2-butene molecules
Horizontal or vertical rotation of unsymmetrical starting material can result in products that are structural isomers or stereoisomers [ enantiomers, diastereomers etc.].
Example:
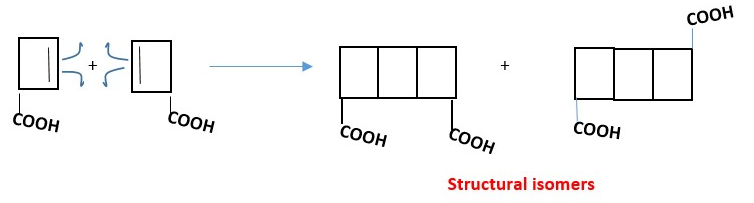
[4 + 2] cycloaddition reactions:
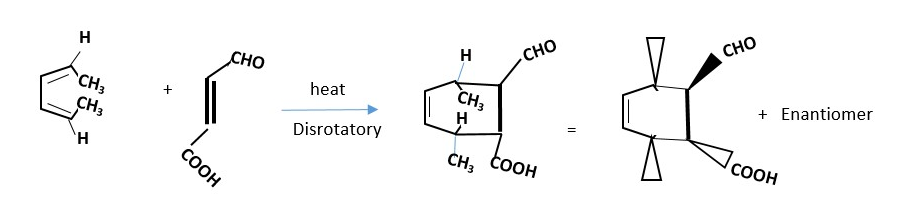
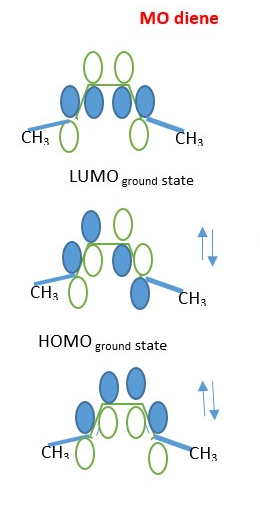
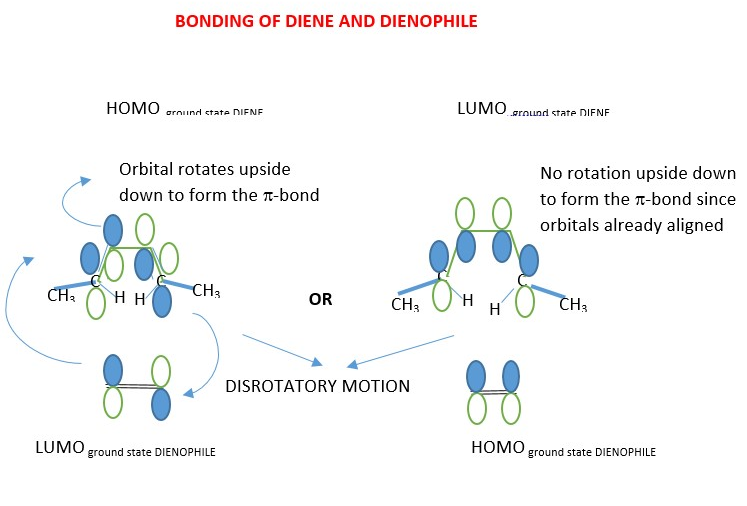
4 + 2 cycloaddition reactions are THERMAL because the ground state HOMO p-electron frontier orbitals in diene overlaps in the same phase in Disrotatory manner with ground state LUMO p-electron frontier orbitals of the dienophile to form sigma bonds. The opposite is also true whereby the ground state HOMO p-frontier orbitals of dienophile forms same phase overlap with ground state LUMO p-frontier orbitals of the DIENE.
Determine how the STEREOCHEMISTRY of the product is established in the thermal reaction shown below:
Assign Dash wedges to groups is pointing downwards but Bold wedge to groups pointing upwards after motion (disrotatory) of the orbitals.
The p-orbitals move in disrotatory motion to form the sigma bonds and p-bond in the product of the reaction between DIENE and DIENOPHILE. The ground state HOMO of diene react with ground state LUMO of dienophile or the ground state LUMO of diene reacts with ground state HOMO of dienophile gives the same product.
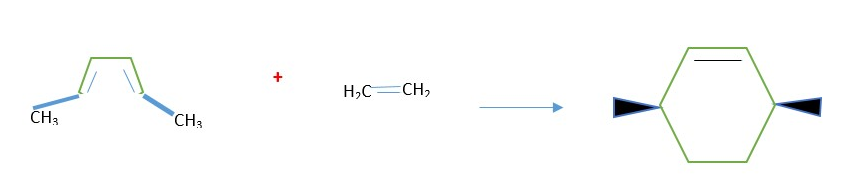
An example of a 4+2 cycloaddition reaction is Diels Alder (DA) reaction.
Dienophiles with electron withdrawing groups attached to the C=C bonds are good candidates for DA reactions. However, there are some cases where the DIENE has electron withdrawing groups attached. Dienophile contributes 2 p-electrons while Diene contributes 4 p-electrons, therefore DA reaction is a 4 + 2 cycloaddition reaction.
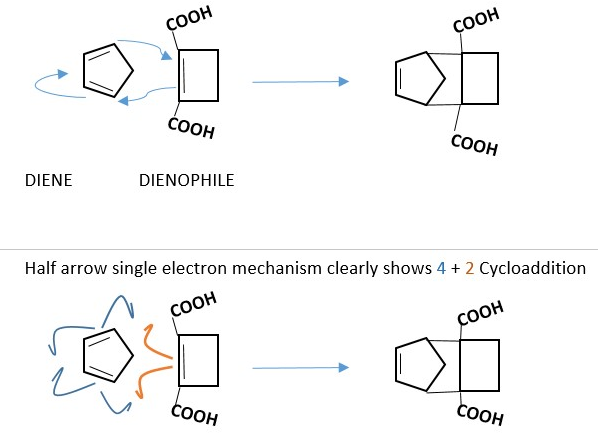
There are other electron cycloaddition reactions which are different from DA reaction depending on number p-electron pairs involved and reaction conditions such as heat or light or darkness.
Whether you use the full arrow method or the half arrow, you should get the same answer. The actual reaction occurs when the molecules are close enough to form bonds. This also require the two species to approach each other in the correct position or orientation to form the bond. The two double bonds in the DIENE must be in a SYN orientation at a bonding distance from the dienophile.
The DIENE must be in a syn-endo-cis orientation to react with the Dienophile and form product. Wrong orientation of molecules leads to little or no reaction.

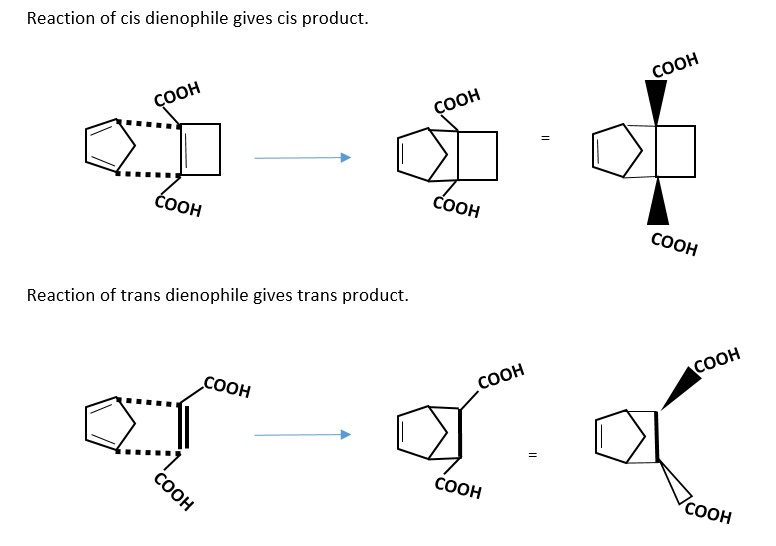
Stereochemistry of the product of obtained from the reactant DIENOPHILE original stereochemistry. A cis will give cis product while trans gives trans product.
Example.
Racemic or other isomeric products form If diene or dienophile is unsymmetrical vertically or horizontally.
Example .
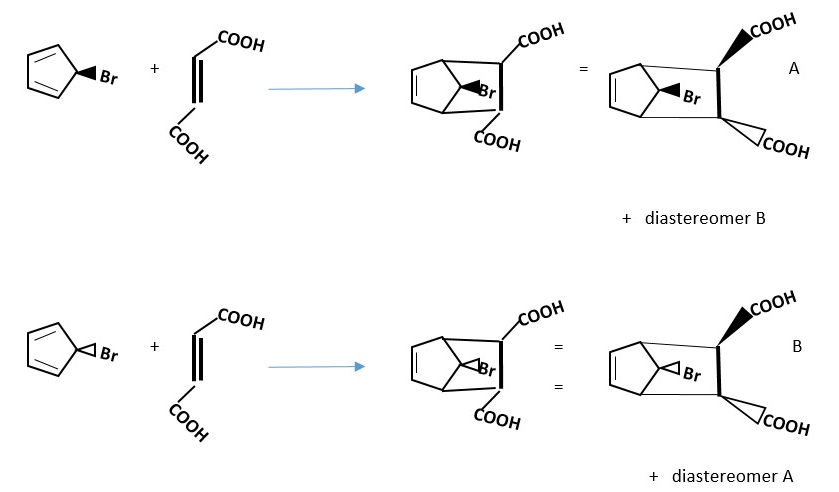
For the reaction below try and figure out the structure of the enantiomer or any other product that will also form.
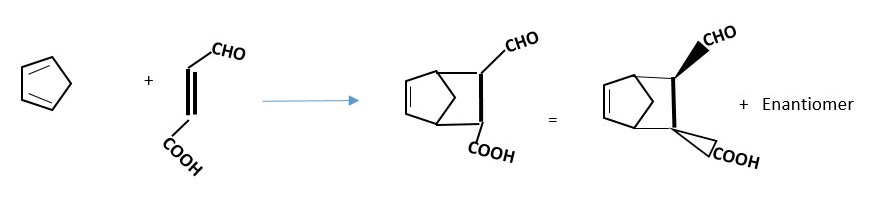
Prove the reaction below will occur by DISROTATORY mechanism to form products and draw the structure of the enantiomer and other possible products.