OXIDATION NUMBERS AND REDOX REACTIONS:
Common Oxidation state numbers: How to calculate oxidation numbers
This refers to the number charge on an atom of an element in the neutral pure elemental form or in a compound.
In most cases it is easy to determine the common oxidation charge of the main group elements in a COMPOUND by using the periodic table. The elements in group one to four have their common oxidation charge based on the group number. Hydrogen is in group 1 on the periodic table so its common oxidation charge is +1 in most chemical compounds excerpt in hydrides when it is -1. Lithium and Sodium and Potassium all have charge of +1 in chemical compounds [ .e.g. oxidation charge of Sodium is +1 in NaCl]. Magnesium is in group 2 on the periodic table so its common oxidation is +2 in compounds [e.g. Magnesium is +2 in MgO].
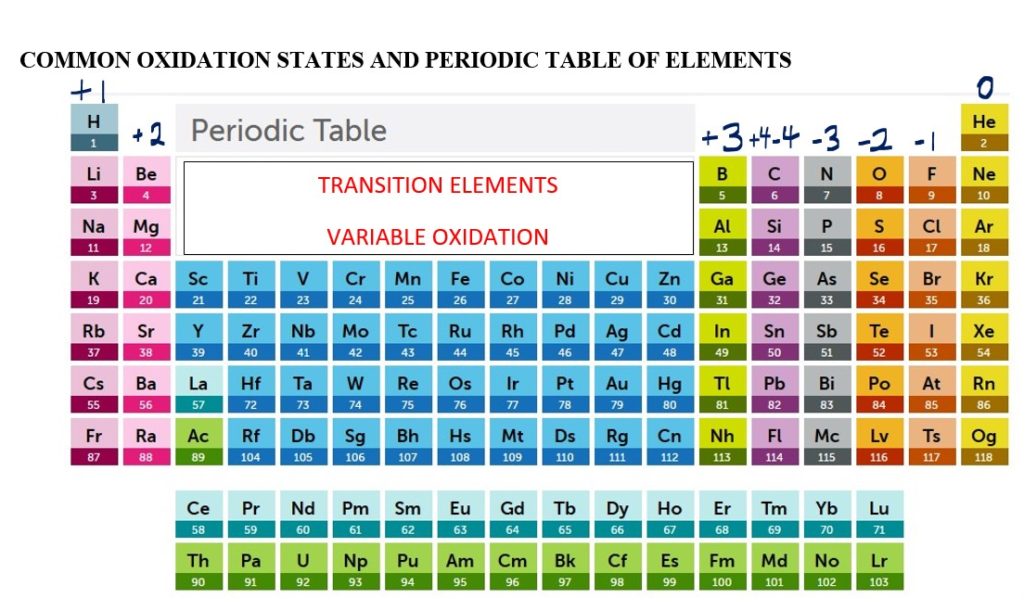
The elements in group 4 have common oxidation charges which ranges from -4 to +4 in most compounds. After group 4, the sign of the common oxidation charge changes to (-) negative values and becomes ; common oxidation charge number = group number- 8. Oxygen is group column 6 so the common oxidation charge for oxygen = 6-8 = -2. [(Excerpt in peroxides ( e.g hydrogen peroxide H2O2 when oxygen is -1 charge)]. Oxygen has oxidation number of -2 in MgO. Chlorine is in group 7 so its oxidation number = 7-8 = -1 [e.g. Cl is -1 in NaCl].
The transition metal elements usually tend to have variable oxidation numbers in compounds. E.g., Copper is usually +1 oxidation charge as in Cu2O ,Copper (l) Oxide, or +2 oxidation charge as in CuO ,Copper(ll)oxide. Iron is usually +2 as in FeO, Iron (ll) oxide or +3 oxidation charge as in Fe2O3 Iron (lll) oxide.
The number oxidation charge in the PURE ELEMENTAL FORM is ZERO. The oxidation charge of Sodium (Na) in pure solid Sodium metal is zero. Hydrogen in (H2 molecule) (Hydrogen gas) , Oxygen in the form of O2 (oxygen gas) or O3 (Ozone). Sulfur in S2 and solid S8 as well as Nitrogen in N2 all have zero oxidation charge.
Total oxidation state of a compound is zero since a compound is a neutral species and the sign and number of the charges must balance out to ZERO. Therefore, SUM of the charges of the atoms in a compound equate to zero.
e.g. Calculate the oxidation charge of Manganese (Mn) in MnO , MnO2 and in KMnO4
Mn + [O] = 0 , Mn = 0-[O] Mn = 0-2 = +2 …….. Charge of Mn in MnO
Mn + 2 x [O] = 0 , Mn = 0 -2[O] , Mn = 0-2x-2 = 0-(-4) = +4 ……… Charge of Mn in MnO2
K + Mn + 4x[O] = 0 , 1 + Mn + 4x[-2] = 0 , Mn = +7 ………. Charge of Mn in KMnO4
Calculate oxidation number of CARBON in CO, CO2
C + [O] = 0 , C = 0-[O] C = 0-(-2) = +2 …….. Charge of C in CO
C + 2 x [O] = 0, C = 0 -2[O], C = 0-2[-2] = 0 – [(-4)] = +4 ……… Charge of C in CO2
The oxidation number charge of all the atoms in an ION is equal to the charge on the ion.
Na+ =+1 , Cr3+=+3 , O-2 = -2
This means the oxidation number charge of a GROUP of atoms forming an ION is equal to the to the charge on the ion.
E.g. Calculate oxidation number of CARBON in CO3-2 and C2O4-2
C + 3x[O] = -2 , C = -2 – 3[O] , C = -2-(3x(-2)) = +4 ……….Charge of C in CO3-2
2C + 4x[O] = -2 , 2C = -2 – 4[O] , 2C = -2-(4x(-2)) = +6 , C=+3 ……….Charge of C in C2O4-2
Calculate oxidation number of SULFUR in SO3-2 and SO4-2
S + 3x[O] = -2 , S = -2 – 3[O] , S = -2-(3x(-2)) = +4 ……….Charge of S in SO3-2
S + 4x[O] = -2 , S = -2 – 4[O] , S = -2-(4x(-2)) = +6 ……….Charge of S in SO4-2
Calculate oxidation number of Mn in MnO4-1
Mn + 4x[O] = -1 , Mn = -1 – 4[O] , Mn = -1-(4x(-2)) = +7 ……….Charge of S in SO4-2
Oxidation state number of Carbon in organic compounds can vary depending on the functionality.
Oxidation number of carbon in methane CH4: 4[H] + C =0 , 4[+1] + C =0 , C = 0-4 = -4
Oxidation number of carbon in ethane C2H6 : 6x[H] + 2C =0 , 6x[+1] + 2C =0 , C = -6/2 = -3.
Now lets calculate the oxidation number of carbon in butane C4H10 :
10x[H] + 4C = 0, 10 x [+1] + 4C =0 , C = -10/4 = -2.5
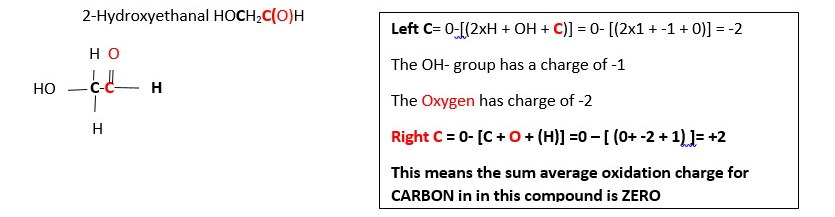
this means not all the carbons have the same oxidation state charge in this compound so expand the structure. This shows two different carbons: the terminal carbons (in pink color) and the middle carbons (black). Each These do not have the same oxidation numbers because they are not bonded in the same way. Terminal carbons are bonded to three hydrogens, and one carbons. The middle carbons are bonded to two carbons and two hydrogens.
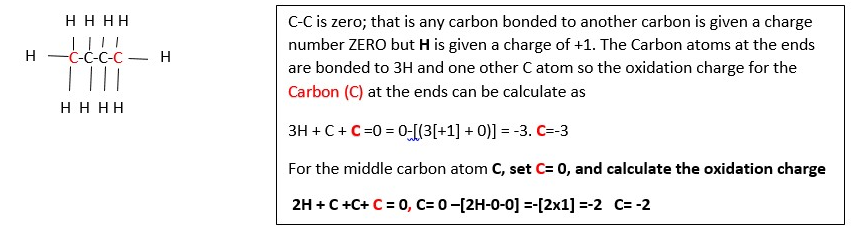
Calculate the oxidation number of the carbons highlighted (black and red) in the following examples ;
Redox reactions
This occurs when one reactant is oxidized or reduced in a chemical reaction. Oxidation is accompanied by loss of electrons OR increased positive charge or oxidation number. Reduction is accompanied by gain of electrons or decreased positive charge or oxidation number. For example, in the chemical reaction of burning of hydrogen in oxygen to form water ; 2H2 + O2 = 2H2O .
The oxidation state of hydrogen changes from zero in H2 to +1 in H2O . However, the oxidation number of oxygen changes from zero in O2 to -2 in H2O. Therefore, hydrogen is oxidized but the oxygen is reduced. The causative agent is H2 molecule because it caused the O2 molecules to be reduced. This is called reduction step and the H2 is called the reducing agent. On the other hands, the O2 molecule has caused the H2 to be oxidized to form water. This is called oxidation step and the O2 is called oxidizing agent.
Examples of redox reaction equations and how to balance them.
- Burning Sodium metal in Chlorine gas to form NaCl
Na + 1/2Cl2 _________________________> NaCl [ unbalanced raw equation]
Redox half equations: This means we have to divide the equation into two parts (Reduction half equation and Oxidation half equation) and use the oxidation charge calculation to identify which element in the reactants (left) is oxidized or reduced to form product (right).
Na _______________> NaCl
1/2Cl2 ________________> NaCl
Redox Half reaction equation for Sodium:
Na_____________>NaCI…………..equation 1… oxidation ……. [Sodium has a zero-oxidation charge in sodium metal (Na) but has +1 charge in NaCl so we have to add 1e- to the right side to balance out the oxidation charge to equal zero on the left side] This gives;
Na-_______________> NaCl+1e-
[The number of sodium material is balanced out in this half equation so we can proceed].
Redox Half reaction equation for Chlorine:
1/2Cl2 ________________> NaCl………. equation 2 ….. reduction …… [Chlorine has a zero charge in Cl2 but has -1 charge in NaCl so we have to add 1e- to the left side of this half equation to balance out the oxidation charge and make it equal to the right side.]This gives;
1/2Cl2 +1e- ________________> NaCl [We have to rewrite the second equation by multiplying through by 2 to remove the fraction. This gives;
Cl2 +2e- ______________> 2NaCl ………. equation 2a
The number of electrons must balance out to zero. The number of electrons lost in the oxidation step must equal the number gained in reduction step and all charges must balance out.
This means we have to multiply through equation 1 by 2 to make the number of electrons equal to that in equation 2a. This gives;
2Na _____________> 2NaCl +2e-
Both charge and materials in the equations (1 and 2a) is balanced out so we can add them and cancel out (or subtract) materials that are the same on opposite sides of the equation. This will give the answer as ;
Cl2 + 2Na_____________> 2NaCl
NOTE: [ Both half reactions have the same product of 2 moles of NaCl so we have to maintain 2NaCl and do not add them up to make 4NaCl ]
EXAMPLE 2.
Burning magnesium metal in oxygen gas to form magnesium oxide
Mg + O2______________> MgO [unbalanced raw equation]
Redox Half reaction equations:
- Mg___________>MgO
- O2 _____________> MgO
Charge of Mg is +2 in MgO but charge of Oxygen is -2 in MgO. Therefore we write
Redox Half reaction equation for Magnesium (Mg):
Mg ___________> MgO …………1..Oxidation [oxidation charge of Mg is zero in Solid Mg metal but becomes +2 charge Mg+2 in MgO.] Therefore, we have to add 2e- to right side to balance out charges to equal zero Mg on the left. This gives ;
Mg ___________> MgO + 2e-
Redox Half reaction equation for Oxygen:
1/2O2 –__________>MgO ……..2..Reduction…[Oxidation charge of Oxygen is zero in O2 but becomes -2 charge in MgO]. Therefore, we have to add 2e- to left side to balance out charges to equal the -2 charge on the right]. This gives :
1/2O2 + 2e- –__________>MgO
Now both charge and materials are balanced in each half reaction, lets add the two equations and cancel out or subtract materials that are the same on opposite sides. This gives;
Mg+ 1/2 O2__________________> MgO ……………….[only one MgO appears on product side because both half equations give the same product MgO].
EXAMPLE 3
Exothermic reaction of burning hydrogen gas in oxygen gas to form water.
H2 + O2 __________________________> H2O [unbalanced raw equation]
Redox Half equation for Hydrogen:
H2 ____________________>H2O ……OXIDATION… [Oxidation charge of H is zero in H2 but is +1 in H2O . Therefore, we have to add one electron to the right side but there is two H so we add 2 electrons to right side instead of one. This gives;
H2 __________>H2O + 2e- [Oxidation charge is balanced out (0=0) and the number of hydrogens is also balanced]
Redox Half equation for Oxygen:
O2 ________>H2O………..REDUCTION.. [Oxidation charge of oxygen is zero in on left side (O2) but is -2 on right side (in H2O) so we have to add two electrons to the left side to balance the oxidation charges. This gives ; O2 + 2e- _________>H2O
However, there is 2 oxygens on the left side, so we have to multiply the number of electrons by 2 . Also multiply the H2O on right side by 2]. This gives;
O2 +4e- _______________> 2H2O [Charge is balanced now and the number of oxygens is also balanced now.
We must multiply the first equation by 2 to make the number of electrons equal in both half equations. This gives;
2H2______________> 2H2O + 4e- [ Hydrogens and charge are now balanced out in this half equation]
Now let’s add the two equations and cancel or subtract items that appear the same at opposite sides. This gives;
2H2 + O2_______________> 2H2O
[ water is the product of the two half reactions, so we don’t have to add up water but just take one 2H2O. The equation is balanced now.
EXAMPLE 4.
Conversion of hydrogen gas to nitrogen gas and water by reacting with nitric oxide
H2 + NO _____________ > H2O + N2 [unbalanced equation]
Redox Half equation for hydrogen:
H2 ___________> H2O …..oxidation .. [oxidation charge of hydrogen is zero in H2 on left side but is +1 in H2O on right side. Therefore, we have to add 1e- to right side to balance out charges to equal the zero charge on the left]. This gives;
H2 ______________> H2O + 1e-] ………..However, there is 2 hydrogens, so we have to multiply 1e- by 2. This gives ;
H2 __________> H2O + 2e- ………….1.
Redox Half equation for Nitrogen:
NO ___________ > N2……. Reduction …. Oxidation charge of nitrogen is +2 in NO on left side but is zero in N2 on right side. Therefore we have to add 2e- to left side .
NO+2e- _____________> N2] ……. Charges balance out to zero but number of nitrogens are not balanced , so the left side NO and electrons (2e-) must be multiplied by 2. This gives ;
2NO +4e- _____________ > N2………….. since this equation has 4 electrons, we multiply the first equation by 2 to make the number of electrons equal in both redox equations. This gives;
2H2 _____________ > 2H2O + 4e- …………..equation 2.
Now we can add the two equations 1 and 2 to get the final overall balanced equation.
2NO +2H2 _________________> N2 + 2H2O.
EXAMPLE 5
Combustion of methane in BASIC medium to give carbon dioxide and water.
C2H6 + O2 _____________>CO2 + H2O
Half equations oxidation state of hydrogen does not change in the reaction but carbon and oxygen have changes in their oxidation state so we focus on C and O.
Redox Half equation for Carbon:
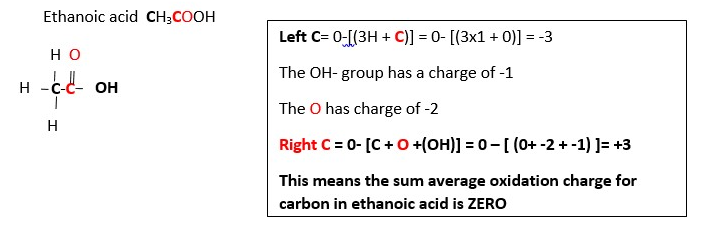
C2H6_____________ > CO2 ………. Oxidation half equation…[Carbon is -3 in C2H6 but is +4 in CO2 IMPLIES seven electrons 7e- must be added to right side to balance out the oxidation charge.]
C2H6 __________> CO2 +7e-…….. Charges are balance out but not the number of carbon material; therefore, we have to multiply the right side by 2 to balance the carbons and charges. This gives; C2H6 ______________> 2CO2 + 14e-
In Basic medium, we have to use OH– ions to balance out the charges that appear in the reaction equation ( I will call them VISIBLE CHARGES).
In this case we have to add 14OH- ions to the left side to balance out the charge then add water (7H2O) molecules to the opposite side . For every 2 mole OH- , you can add one mole of water to the opposite side and check for material balance. You can make adjustments where necessary. Therefore we get. C2H6 +14OH– ______________>2CO2 + 14e- + 7H2O ……..1….. [CHECK: not balanced. We need to add 3 more moles of H2O to balance it]. This gives;
C2H6 +14OH– ______________> 2CO2 + 14e- + 10H2O ……..1
Redox Half equation for Oxygen:
O2 ______________> H2O …………. reduction ..[ Oxygen is in zero oxidation state in O2 but is -2 charge in H2O, therefore we must add 2e- to left side.] This gives;
O2 +2e-___________>H2O ……[ material number of oxygens is not balanced (2:1), so we have to put 2 in front of water to get 2:2] Thus, we get ;
O2 +2e-____________>2H2O ……[The oxidation charge of oxygen in H2O is -2, this doubles the charge on the right side to -4 so we have to replace the 2e- on the left side with 4e-]. This gives;
O2 +4e- ____________>2H2O …..There is -4 visible charge on the left side but ZERO visible charge on right side. We are working in a basic medium so we have to balance the -4 charge on the left side by adding 4OH– to the right side and then add 2H2O to left side . This gives;
O2 +2H2O +4e-__________>2H2O + 4OH–……[Check : Charge is balanced but materials (number of oxygens) are not balanced. We have to add two more water molecules to the left side to balance it] . This gives;
O2 + 4e- + 4H2O ___________> 2H2O + 4OH– Now we have to make the number of electrons equal in both equations, so we have to multiply through this equation by x 3.5 to get 14 electrons. We get;
3.5O2 + 14e- + 14H2O ______________> 7H2O + 14OH– …….2
We add equation 1 and 2 to get the overall final balanced equation; Add items that are the same on the same side but subtract items that are the same on opposite sides.
The overall balanced equation is ;
C2H6 + 3.5O2______________> 2CO2 + 3H2O [mutiply through by 2 to remove the decimal number]
2 C2H6 + 7O2 _____________> 4CO2 + 6H2O
NOTE: We can also solve the same reactions equation and get the same answer if we were working in an ACID medium.
In acid medium we use hydrogen ions [ H+ ] to balance out charges that appear in the reaction equation (visible charges).
For example, in the previous example above , when you reached the stage ;
Redox Half equation for Carbon…. acid medium
C2H6___________>2CO2 + 14e- [Total visible charge on the left is ZERO but the right side is -14 so we have to add 14H+ to the right-side balance out charge and then add half equivalent number moles of water to opposite side and check the equation to see if the equation is balanced]. This gives ;
C2H6 + 7H2O__________ >2CO2 + 14e- + 14H+ ………..1 [Charges are balanced but not the materials. Careful check shows we have to reduce the number of moles of water from 7 to 4 ]. This gives;
C2H6 + 4H2O________> 2CO2 + 14e- + 14H+
Redox Half equation for Oxygen……acid medium
O2 +4e- ____________>2H2O [Total visible charge at right side is zero but is -4 on the left so we have to balance this by adding 4H+ to the left side and then add 2H2O to right side]. This gives;
O2 +4e- + 4H+___________> 2H2O + 2H2O [ Upon careful examination, the additional 2H2O is not necessary since the equation is already balanced with the 4H+] so we get ;
O2 +4e- + 4H+_____>2H2O ………….2 [ we have to multiply this equation by 7/2 or 3.5 to make the number of electrons equal in both half equations]
3.5O2 +14e- + 14H+_____________> 7H2O ……………2a
Add equations 1 and 2a to get overall balanced equation.
C2H6 + 3.5O2 ________________> 2CO2 + 3H2O …..multiply by 2 to remove decimals. This gives;
2C2H6 + 7O2 ________________> 4CO2 + 6H2O
EXAMPLE 6
Potassium permanganate dissolves in a basic solution to form manganese dioxide
KMnO4 + Na2C2O4(aq) _________> MnO2 + Na2CO3 (aq) [unbalanced raw equation]
Redox half equation for Manganese (Mn):
MnO4– _________> MnO2 …….REDUCTION…1…[Oxidation charge of Mn is +7 in KMnO4 on the left but is reduced to +4 in MnO2 on the right . Therefore, we have to add 3e- to left side to balance charges.] This gives;
MnO4– + 3e- _________> MnO2 [materials (in terms of Mn) in equation is balanced now so we can proceed now]
The reaction is in Basic medium, so we have to use OH– ions to balance out the visible charges. Total charge on the left side is -4 now so we have to add 4OH- to right side to balance out the charge and then add some 2H2O (water) to the left side to balance hydrogen and oxygens. This gives;
MnO4– + 3e- + 2 H2O _________> MnO2 + 4OH- [ both charge and materials are balanced out now]…….1a
Redox half equation for CARBON (C):
Na2C2O4(aq) is just 2Na+ + C2O4-2 (OXALATE ION). The 2Na+ ions are just spectators, so we can just use the oxalate ion to write the equation.
C2O4-2 _________> CO3-2 …..OXIDATION…2…[ Oxidation charge of Carbon changes from +3 in C2O4-2 ion to +4 in CO3-2 ion. Therefore we have to add 1e- to right side to balance out oxidation charges. This gives;
C2O4-2 _________> CO3-2 + 1e-
Now we have to balance the materials (number of carbons) by multiplying the right side by 2 to balance out the carbons. This gives;
C2O4-2 _________> 2CO3-2 + 2e- [carbons are balanced so we can proceed.]
[ now the total charge on left side is -2 but the right side has 2(-2) + -2 = -6 so we have to add 4OH– to the left side to make the total charge the same as the right side. We need to add some water (2H2O) to the right side to balance the H and O. This gives;
C2O4-2 + 4OH– _________> 2CO3-2 + 2e- + 2 H2O …..2a…..[both charge and materials are balanced now]
We have to make the number of electrons in equation 1a equal to number of electrons in equation 2a .
MnO4– + 3e- + 2 H2O _________> MnO2 + 4OH-………1a
C2O4-2 + 4OH- _________> 2CO3-2 + 2e- + 2 H2O …….2a
1a x 2 : 2MnO4– + 6e- + 4 H2O _________> 2MnO2 + 8OH-
2a x 3 : 3C2O4-2 + 12OH- _________> 6CO3-2 + 6e- + 6 H2O
Add both equations to get overall balanced equation: [Cancel out or subtract the same items on opposite sides. Add same items on same side]
2MnO4– + 3C2O4-2 + 4OH- _________> 2MnO2 + 6CO3-2 + 2H2O
EXAMPLE 7
Potassium dichromate converts ethanol to in aqueous acetic acid
K2Cr2O7 + CH3CH2OH __________> Cr3+ + CH3COOH [unbalanced reaction equation]
Redox half equation for Chromium (Cr):
K2Cr2O7 is just 2K+ ions and Cr2O7 -2 ions . K+ ions are just spectator ions so we have to use Cr2O7 -2 ions to write the half equation.
Cr2O7-2 __________> Cr3+ …….REDUCTION…….1… [oxidation state of Cr is +6 in Cr2O7 -2 but is +3 in Cr3+ , therefore we have to add 3e- to left side to balance out the oxidation states. This gives;
Cr2O7-2+ 3e- __________> Cr3+ [oxidation charges are balanced but materials (number of Cr) are not balanced. Therefore, we must multiply right side by 2 to balance out Cr. This means we also have to double the electrons on left side to 6e-. This gives;
Cr2O7-2+ 6e- __________> 2Cr3+ [ now Cr is balanced so we can proceed.]
Acid medium: we have to balance visible charge by using H+ ions ; total visible charge on left side is -8 but total visible charge on right side is 2x 3 = +6 . Therefore, we add 14H+ ions to left side balance out total charges and then add water (7H2O) to the right side to balance the H+ ions. This gives;
Cr2O7-2+ 6e- + 14H+ __________> 2Cr3+ + 7H2O [both charge and material are balanced now]……….1a
Redox half equation for Carbon (C):
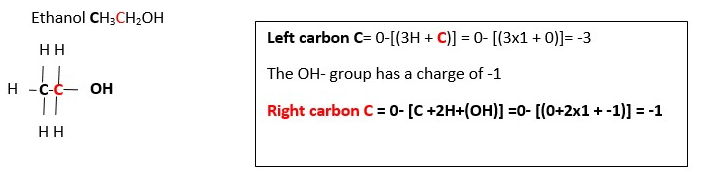
CH3CH2OH ____________> CH3COOH ………OXIDATION …..2..[ Oxidation charge of the functional group CARBON (Carbon in pink color) is -1 in CH3CH2OH but is +3 in CH3COOH. Therefore, we must add 4e- to right side to balance out oxidation charges. This gives;
CH3CH2OH ____________> CH3COOH + 4e- [ Number of Carbons are balanced so we can proceed]
Total visible charge is zero on left side but is -4 on right side. Therefore we must add 4H+ to right side to balance out the charge . Then add H2O to left side to balance the hydrogens and oxygens. This gives;
CH3CH2OH + 2H2O____________> CH3COOH + 4e- + 4H+ [ Charges are balanced but materials in equation are not balanced]
Careful inspection shows that we have two options to balance the materials in the equation.
1) Keep the moles of water on the left side but double the moles of ethanol on left side and also double everything on the right side. or
2) Reduce the moles of water by half and keep all the others.…[easier].
Either one will give the correct answer; Using the first option gives ;
2CH3CH2OH + 2H2O____________> 2CH3COOH + 8e- + 8H+ …………Eqn 2a [ both charge and materials are balanced now]
Lets make the number of electrons in both half equations equal
1a x 8 : ……………….. 8Cr2O7-2+ 48e- + 112H+ __________> 16Cr3+ + 56H2O
2a x 6:………………….. 12CH3CH2OH + 12H2O____________> 12CH3COOH + 48e- + 48H+
Lets add the two equations to get overall balanced equation. This gives;
8Cr2O7-2 + 12CH3CH2OH + 64 H+ ______> 12CH3COOH + 16Cr3+ + 44 H2O
Divide through by 4 to get smallest whole number of moles of reactants and products;
2Cr2O7-2 + 3CH3CH2OH + 16H+ ______> 3CH3COOH + 4Cr3+ + 11 H2O
DISPROPORTIONATION
Disproportionation: This refers to a redox reaction when one element is reduced and oxidized at the same time. Normally this happens when an element appears in two reactants and all going to form the same product.
e.g. 2H2O + O2 _______> 2H2O2 [The oxidation charge of Oxygen in water is -2 but -1 in H2O2 . At the same time oxygen has zero charge in O2 but is -1 in H2O2] This shows disproportionation of oxygen in the reaction.
The following are disproportionation reactions involving nitrogen; Follow the oxidation state of nitrogen from reactants to products to verify this.
2NO2 __________> NO2 + NO3
2NO2 + H2O ________> HNO2 + HNO3
3HNO2 (aq) __________> HNO3 + H2O + 2NO

