Fuel Production| Carbon dioxide (CO2) emissions
Fuel production green chemistry relates to the production of fuels without byproducts that pollutes the environment. The main concern is carbon dioxide (CO2) gas from fuel production and its effects on the environment . Carbon dioxide (CO2) emissions occur from complete combustion of fossil fuels.
Global warming from CO2 emissions |
Carbon dioxide (CO2) is heavy gas molecule with slower speed. This means it cannot easily escape the earth’s gravitational pull to go into space. Most Carbon dioxide (CO2) emissions will remain trapped in the atmosphere of the planet. Some CO2 is used up by green photosynthetic plants for photosynthesis. Meanwhile, the excess CO2 is much more than the Earth can recycle through green plants photosynthesis.
Carbon dioxide absorbs heat emission from the Earth and return the radiation back to the Earth. This means increased amounts of CO2 gas in the atmosphere will result in increased temperature of the planet. This in effect can cause huge environmental catastrophe such as rise in ocean levels and melting of the polar ice. The focus on fuel and green chemistry comes as a result of trying to find ways to minimize or eliminate CO2 in fuel production.
Fossil fuels : Source of mass CO2 emission into atmosphere |
The problem of global warming from carbon dioxide is a major problem in the world. Most of this comes from use of fossil fuels as well as production of the fossil fuels such as oil and natural gas. Modern automobile engines are equipped with filters and converters to capture CO2 . However it will take time for everyone in every country to have this technology in their cars. This means, industries that use fossil fuels as energy source will also need some technology to capture and convert CO2 gas .
Drilling and processing of fossil fuels, also involves burning of fuel that releases CO2 gas directly into the atmosphere. This makes it necessary to seek other ways to obtain fuels in way that will result in zero emission of CO2 gas . Detailed information on the use of energy around the world and the production of fossil fuels and CO2 is found in international energy outlook paper.
Solution : Eliminate CO2 emissions in fuel production |
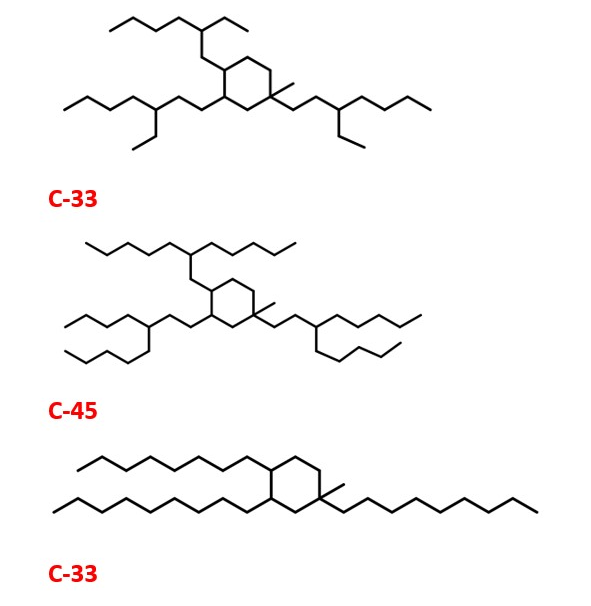
Organic compounds namely ethanol, 1-butanol and acetone from fermentation of sugarcane and other processes are very important compounds . They can be a good starting material for the synthesis of Jet fuels and lubricants which have superior qualities compared to existing ones. This is mainly through alkylation , trimerization and reduction. PINAS , vol 112, pp. 7565. Methyl ethyl ketones can also be made from microbes . Industrial & Engineering Chemistry Research, 52(1), 56-60. The process is a potential alternative for obtaining fuels through renewable and eco-friendly methods. This is due to zero emission of green house gases such as CO2. This is good for carbon capture and reduction of green house gas emissions and as a result uses the fuel production green chemistry approach.
This method could also pave the way for environmentally friendly way of making fuels and lubricants for automobiles . Synthesis of Transportation fuels : Chev. Rev. 2006, 4044.The structures are also shown here. Ref: PINAS , vol 112, pp. 7565
JET FUEL
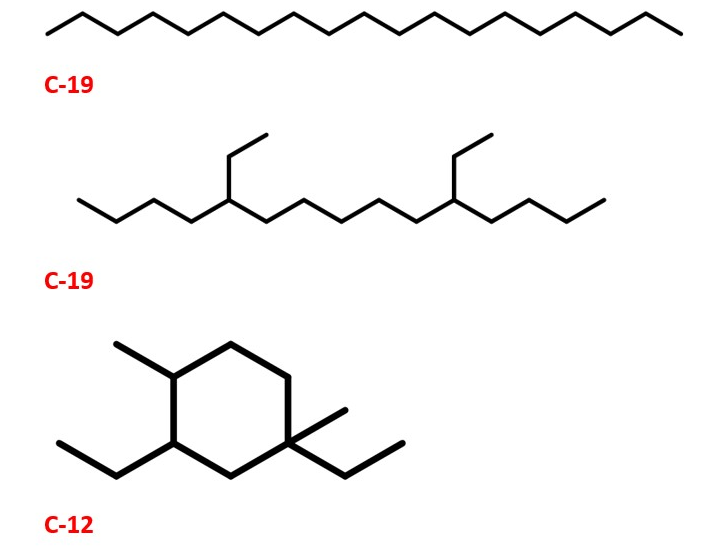
LUBRICANTS