ELECTROCHEMISTRY
Electrochemistry
Electrons flowing from one end to another generates a current. Chemical reactions that involve redox reactions can be separated into containers called electrodes. The solution of charged particle is usually called an electrolyte. Negative charged particles are called Anions, and the positive charged particles are called Cations. Moreover, electron flow from one electrode to the other electrode be measured by using a voltmeter. Electrodes have charges due to electron flow.
The negative electrode is called a cathode since they receive cations. However, the positive electrode is called an anode since they receive anions. This helps charges to balance out in the whole system while generating electricity. Sometimes there is a bridge of salt connected to across the system to balance out the charges. This is called the Salt bridge. The amount of electricity produced can be calculated from the moles of substance used up or produced from the electrochemical reaction.
Electrochemical / Activity series
A + B ________> A + + B– , this means element A has given an electron to element B to become A + and B- . A has reduced B . Therefore, A is reducing agent [it is oxidized to form product A+]. On the other hand, B has taken an electron from A. this means B oxidized A . Therefore, B is oxidizing agent [ it is reduced to form product B-] .
Transfer of electrons from a place of higher oxidation potential to a place of lower oxidation potential creates a potential difference. In other words, electrons flow from a lower reduction potential to a higher reduction potential. The flow of electrons in one direction is a force [aka electromotive force ( e ) = emf ] that creates electrical energy or electricity that can be measured.
The power of an element to reduce or give electrons to Hydrogens ions to form hydrogen gas and become OXIDIZED at 25oC is called standard oxidation potential.
Positive values mean reaction is feasible in forward direction as written.
E.g. 2A + 2H+ _________> 2A + + H2 (gas)
Negative value means reaction will occur in reverse direction .
2A + + H2 (gas) _________> 2A + 2H+
Electrochemical / activity series [ability of an element to be oxidized by hydrogen ions]
2K + 2H+ ___________> 2K+ + H2 (g)…………2.93V
2Al + 6H+ ___________> 2Al3+ + 3H2 (g)……..1.66V
Zn + 2H+ ___________> Zn2+ + H2 (g)…………0.76V
2Pb + 2H+ ___________> 2Pb+ + H2 (g)………..0.13V
e- + 2H+ ___________> H2 (g) [standard]…… .0.00V
2Cu + 2H+ ___________> Cu2+ + H2 (g)………-0.34V
2I- + 2H+ ___________> I2 + H2 (g)……………-0.53V
2Fe2+ + 2H+ ___________> 2Fe3+ + H2 (g)……..-0.77V
2F- + 2H+ ___________> F2 + H2 (g)………-2.87V
The element of higher potential can reduce an element of lower potential for a chemical reaction to occur in the direction shown.
E.g. 2Mg + O2 _______> 2MgO [ reaction occur in forward direction because of huge difference in oxidation potential of magnesium and oxygen]
Use a table of electrochemical/activity series to predict if the following reactions will occur as written?
K+ + Li ________> K + Li+
K + Li+________> K+ + Li
KCl + Li ______> K + LiCl
Sn2+ + Cu 2+ ________> Sn4+ + Cu
Sn4+ + Cu ________> Sn2+ + Cu 2+
SnO2 + Cu _____> SnO + CuO
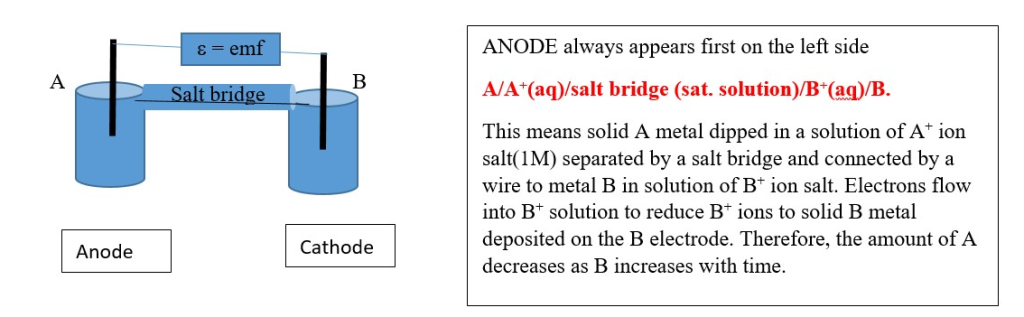
ELECTROCHEMICAL CELL DIAGRAM AND NOTATION
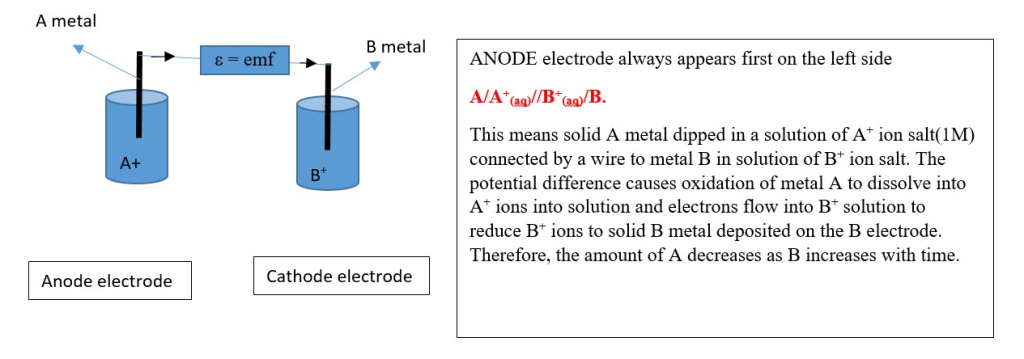
Junction potentials will decrease the overall emf. A salt bridge can be introduced to minimize junction potentials in both electrodes.
Half cell reactions
ANODE: [oxidation] A _________> A + + e- ……….emf = X
CATHODE: [ reduction] B + + e- ________> B ………..emf = Y
Overall reaction: A + B+ ________> A+ + B ……. Cell emf = emf[anode] + emf[cathode] = X + Y
Cell emf = [RT/nF]Ink.
=[0.059V/n] logK
NOTE : The stoichiometry does not affect the value of the electric potential difference.
e.g Electrochemical cell made of aluminum anode and hydrogen cathode [hydrogen with Pt inert metal].
The Pt does not affect the cell potential but allows electrons to flow into solution to reduce H+ IONS to H2 gas.
[Al/Al(NO3)3(1.0M)//HNO3(1.0M)/H2/Pt]
Anode reaction: Al + Al(NO3)3 (aq) ______> Al3++ 3NO3– + Al3++3e-
We don’t have to write all this but just show the species oxidized at the anode and forget about inert counter ions. Solid Al is oxidized to aqueous Al3+ ions.
Al _____> Al 3++ 3e-…………1.66V
Cathode: H+ + NO3 + e- ______> H2(g) + NO3–
We don’t have to write all this but just show species reduced (H+) and forget about counter ion NO3–
H+ (aq) + e- ______> H2(g) ……0.00V
[need to multiply left by 2 to balance H but multiplying does not affect the reduction or oxidation potential.]
2H+ (aq) + 2e- ______> H2(g) ……0.00V [DO NOT MULTIPLY]
Al _____> Al 3++ 3e-…………1.66V ………..equation1
2H+ (aq) + 2e- ______> H2(g) ……0.00V……equation 2
Multiply equation 1 by 2 and equation 2 by 3 to balance the number of electrons in the anode and cathode reactions.
2Al _____> 2Al 3++ 6e-…………1.66V [do not multiply]………..new equation 1
6H+ (aq) + 6e- ______> 3H2(g) ……0.00V……………………….new equation 2
Add to get overall reaction:
2Al(s) + 6H+ (aq) _____> 2Al 3+ + 3H2(g)
Overall emf = 1.66 + 0.00 = 1.66V
NOTE: Using oxidation potential values we add the potential values [emf =Anode + Cathode].
However, if your table in your textbook is in reduction potential values and you don’t want to change the sign of the anode potential, then you have to subtract ANODE potential from CATHODE potential, [emf= Cathode – Anode]
Reaction equilibria and emf measurement.
G = Go + RTInQ , where Q is reaction quotient
ΔG = ΔGo + RTInQ .
-nFemf = -nFemfo + RTInQ .
emf=emfo – [RT/nF]InQ.
=emfo – 0.059V/n[logQ] …. Nernst equation.
K or Q can be ion concentration [in molarity] or gas pressure [in atm or bar ]. Solids are omitted.
K or Q = [product]/[reactants]
e.g. A(s) + B(s) +H2O _________> A+ (aq) + B–(aq)
emf = emfo – 0.059V/n[log[A+][B–]………
n is number of electrons involved in the balanced chemical reaction. Solid A and B are omitted in the Nerst equation.
Therefore, emf=emfo – 0.059V/[log[A+][B–],
Standard reduction potentials or standard oxidation potentials (emfo) are given in tables in your textbook. The values are the same but opposite in sign.
For the cell reaction, A(s) + 2B+(aq) _____> B2(gas) + A+2(aq)
Cell notation is A/A+2/B+/B2
emf=emfo – 0.059V/2[log([A+] (PB2)/[B+]2)] , value of n = 2
where PB2 is the pressure of gas B2 .
Example :
Write the expression of the emf (e) under different conditions other than 1atm and 1.0M concentrations for the example above.
2Al(s) + 6H+ (aq) _____> 2Al 3+ + 3H2(g)
emf = emfo – [RT/nF]InQ ,
where n=6 and Q=[product]/[reactants]
emf = emfo – [RT/nF]In[([2Al 3+ ]2 PH23 ) / ([H+]6[Al])],
Al solid is omitted since activity or concentration is taken as 1.
emf = emfo – [RT/6F]In[([Al 3+ ]2 PH23 ) / ([H+]6)]
emf = 1.66 – [RT/6F]In[([Al 3+ ]2 PH23 ) / ([H+]6)]
Calculate the pH of the nitric acid if the cell emf is 1.02V and the concentration of Al(NO3)3 is 1.89M and the pressure of the gas is 2.5 atm.
emf = 1.66 – [RT/6F]In[([Al 3+ ]2 PH23 ) / ([H+]6)]
1.02V =1.66V – (0.059V/6) x [log([([Al 3+ ]2 PH23 ) / ([H+]6)])
1.02V =1.66V – (0.059V/6) x [log([([1.89M ]2 X (2.5atm )3 / ([H+]6)])
(0.059V/6) x [log([([1.89M ]2 X (2.5atm)3 / ([H+]6)]) = 1.66V – 1.02V = 0.64V
[log([([1.89M ]2 X (2.5atm )3 / ([H+]6)]) = 0.64V x 6 / 0.059V = 65.085
The values in the brackets are already divided by standard units so no units in brackets.
log([([1.89 ]2 X (2.5 )3 / ([H+]6)]) = 65.085
([([1.89 ]2 X (2.5 )3 / ([H+]6)]) = e(65.085 x 2.303) = 1.25 x 1065
([H+]6)]) = [1.89 ]2 X (2.5 )3 / 1.25 x 1065
= 0.000028028
[H+] = (0.000028028)1/6
=0.1743
pH = -log[H+] .
= -log[0.1743] = 0.76
EQUILIBRIUM SITUATION
Equilibrium means K = Q and ΔG = 0
0 = ΔGo + RTInQ
therefore, we can deduce that 0 = emfo – [RT/nF]InQ
emfo = [RT/nF]InQ , or emfo = 0.059V/n[logQ]
from this we can calculate the equilibrium constant since Q=K and then estimate the minimum amount of substance needed to shift equilibrium forward or backwards.

