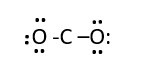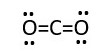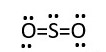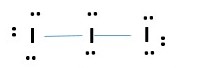Lewis structures, VSEPR, Formal Charges and Resonance Structures
LEWIS STRUCTURES
This is a simplified structure of a compound or ion determined by using the valence electron in the atoms in the compound or ion.
The following rules are useful in writing Lewis structures.
a) Add the total number of electrons in the outermost shell of all the atoms of the compound. If working with an ION, add electrons for negative charge on ion but subtract electrons for positive charge on ion.
b) Atoms are connected by straight lines to show electron pair covalent bonds.
c) Non-bonding electron pairs are placed on each atom to satisfy octet (except duplet for hydrogen atoms).
d) Count the number of electrons in the final structure.
This must be equal to the total sum of the number of outer electrons of the atoms involved (number in a).
e) Remove two lone pairs electrons from two bonding atoms and replace with an additional bond, if there are more electrons than required.
This results in double or triple bonds. Repeat until the number of electrons is equal the number in (a) or (d).
In special cases, some atoms can have less than 8 outer electrons. These are called electron deficient atoms and examples are Beryllium and Boron. However, there are other elements that can have more than 8 outer electrons. This situation is called expanded octet and examples are Sulfur and Phosphorus.
FORMAL CHARGE
Formal charge is the charge on an atom due to the number and the valency of elements bonded to it. It can be calculated as follows.
Formal charge(FC) = Number of electrons in outermost shell [or group number on periodic table] -Number of non-bonding free electrons-Number of bonds attached to the atom. Therefore, formal charge on an atom is not constant but changes on the type of chemical bonding. The total formal charge which is the addition of all the charge on the atoms must equal the charge on the ion (polyatomic ion ) or ZERO in the case of a compound or molecule.
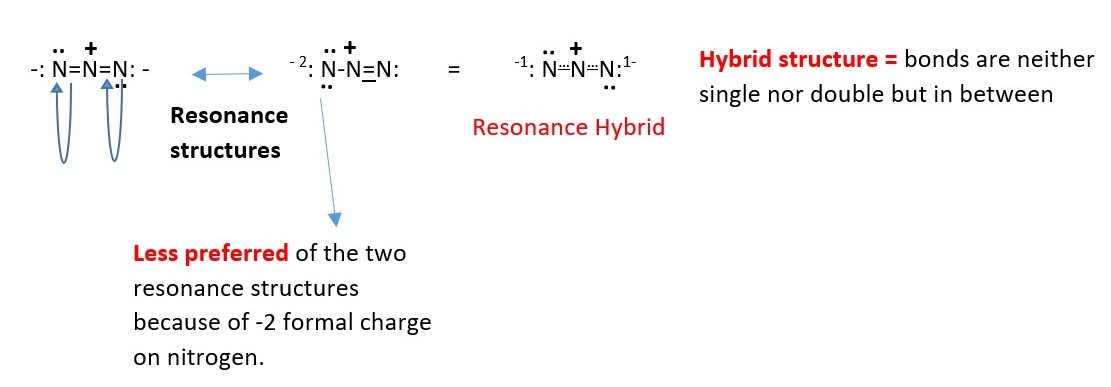
Example : Calculate the formal charge of each nitrogen in Azide ion (N3–)
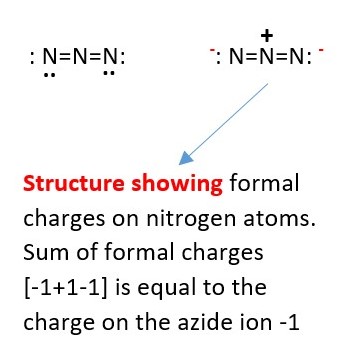
The Nitrogen at the ends have FC = 5-4-2=-1.
However, the central nitrogen FC = 5-4-0=+1.
Therefore, the total charge on the formula is -1+1-1=-1. This is the Azide ion has a total charge of -1.
VSEPR THEORY
Valence Shell Electron Pair Repulsion also known simple as VSEPR theory is very popular and well known. In the VSEPR theory, the electronic geometry includes the shape of structure that is determined by using all electron pairs (both lone and bonding pairs). This SHAPE or geometry is determined by the hybridization of the CENTRAL ATOM in the compound or ion. This is determined as by using the following assumptions.
Each bond counts as ONE electron pair or two electrons.
Every bond between two atoms (single or double or triple) is counted as ONE bond = a shared bonding electron pair= two electrons. e.g . H-H = one bond or one electron pair. C=O is one bond or one electron pair, :C=O is 2 electron pairs, O=C=O is two electron pairs.
HYBRIDIZATION of the CENTRAL atom is determined by the number of electron pairs (Bonding or lone pairs) surrounding the central atom. This determines the electronic shape or geometry of the compound or ion. A few examples electronic geometries are listed below.
2 electron pairs = sp hybridization = LINEAR geometry or shape
e.g. CO, CO2, H2, HF, HCl
3 electron pairs = sp2 hybridization = flat or planar or trigonal planar
e.g. BCl3
4 electron pairs = sp3 hybridization = tetrahedral geometry
e.g. CH4, NH3 and H2O
5 electron pairs = sp3d hybridization = trigonal bipyramid
e.g. I3– , SF5
6 electron pairs = sp3d2 hybridization = octahedral
e.g. XeF6
Electronic structure or geometry of compound depends on hybridization of the central atom.
Molecular geometry is the shape obtained from the electronic geometry without seeing the lone pairs electron cloud. This is the shape of the molecule when you consider only the atoms and the bonds that link them together.
2 electron pairs = sp hybridization = LINEAR geometry or shape = linear molecular geometry.
e.g. CO, CO2, H2, HF, HCl and I3–
3 electron pairs = sp2 hybridization = Molecular shape or geometry can be flat or planar or trigonal planar or Bent or V shape
e.g. BCl3
4 electron pairs = sp3 hybridization = Molecular geometry can be tetrahedral, trigonal bipyramid or bent or distorted tetrahedron or Bent or V shape.
e.g. CH4, NH3 and H2O
5 electron pairs = sp3d hybridization = Molecular shape can be trigonal bipyramid or T-shape or Linear molecular shape.
e.g. SF5
6 electron pairs = sp3d2 hybridization = Molecular shape can be octahedral or square pyramid or square planar.
e.g. XeF6
Lone pair-lone repulsion is stronger that lone pair-bonding pair repulsion which is stronger than bonding pair -bonding pair repulsion.
EXAMPLES OF APPLICATIONS OF LEWIS STRUCTURES, FORMAL CHARGES AND VSEPR THEORY
Always make sure the Lewis structure is correct before proceeding to use the VSEPR theory for the hybridization, electronic geometry and molecular geometry. The correct structure always gives correct values of formal charges.
-
Methane CH4
C is in group 4 has 4 outmost or valence electrons.
H has 1 valence electron, there is 4 H in CH4
Therefore, bond formation requires a total of;
C = 4 e-
H= 4 x 1e-
Total = 8 e-
Draw four lines from carbon at center. Each line represents bonding pair of electrons.
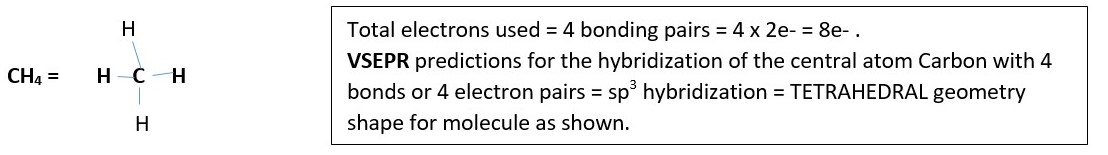
Formal charge of each hydrogen in CH4 is 1-1-0= 0
Formal charge of carbon in CH4 is 4-4-0= 0
-
Carbon dioxide = CO2
C= 1 x 4e-
O= group 6 = 6 valence e- . Two oxygen total = 2 x 6 valence e-= 12 e-
Total electrons needed in CO2 formation =
C= 4e-
O=2 x 6e-
Total= 16e-
Central atom is Carbon atom.
-
Assemble elements and connect with a single line bond O-C-O
-
Each line bond is two electrons, so we need 12 more electrons. Put lone pairs on oxygen to make an octet for each oxygen.
Total electrons used is 6 lone pairs + 2 bonding pairs = 12 +4= 16e- Carbon is not octet, it has only 2 bonding shared electron pairs (4e-) now it needs to be octet. However, the oxygens are octet. Therefore, remove two bonding pairs from each oxygen to form bond with the central carbon. This gives.
In this form all the oxygens and Carbon have octet.
VSEPR prediction for 2 bonds or e- pairs around central atom is sp hybridization which is LINEAR as shown. Therefore, structure is correct.
3. Carbon Monoxide
Carbon monoxide (CO)
C= group 4 = 4 valence e-
O = group 6 = 6 valence e-
Total = 10e-
-
Assemble elements and connect with a single line bond C-O
-
each line bond is two electrons, so we need 8 more electrons. Put lone pairs on oxygen to make an octet for oxygen. The other two electrons are given to carbon.
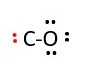
-
Now we have used all 10 e- = one bonding pair + 4 pairs
-
Oxygen has octet but carbon does not. Carbon has only 4 e- now [ one bonding pair(2e-) + 2 lone pairs(4e-)]. Carbon needs 8 e- around it [octet]
-
Remove lone pair from Oxygen to form a bond line with CARBON.
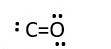
-
Carbon now has 6e- [2 bonding pairs + one lone pair] around it. Still not octet. Oxygen is still octet 8e- [ 2 bonding shared pairs (4e-) + 2 lone pairs (4e-)] . Therefore, carbon needs octet.
-
Remove another lone pair from oxygen to form another bond line with carbon.

-
Now Carbon has octet 8 e- around it [3 bonding shared pair (6 e-) + one lone pair(2e-)]
Oxygen also has octet [3 bonding shared pair (6 e-) + one lone pair(2e-)]
VSEPR prediction for hybridization of the central atom [Carbon or Oxygen] => VSEPR sees only a line and a lone pair around each atom => sp hybridization =LINEAR electronic shape or geometry. The molecular geometry will also be LINEAR as shown in this case above.
Calculate the formal charge each on the Oxygen and Carbon in CO.
EXPANDED OCTET:
Atoms that can have more than 8 electrons around them to be stable.
-
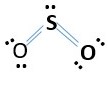
Sulfur dioxide SO2
O = group 6 = 6valence e-
S=group 6 = 6 valence e-
Total number of electrons needed in SO2 formation.
S=6 e-
O = 2 x 6e-
Total = 18e-
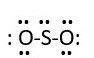
This accounts for 18e- [2 bonding pairs and 7 lone pairs. However, the sulfur central atom does not have octet. Therefore, remove a lone pair from each oxygen to form a bond with the sulfur. This gives.
In this form both oxygen atoms maintain octet, but the sulfur has 10e- around it. This is fine since sulfur can have expanded octet. The VSEPR model prediction of structure is not linear because the central atom [S] has 3 electron pairs around it; that is sp2 hybridization. The electronic geometry is trigonal planar.
Therefore, the Molecular shape or geometry is bent or V shape.
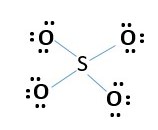
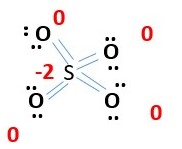
4.Sulfate ion SO4-2
Total number of electrons needed.
S= 6e-
O= 4 x 6e-
Total 30 e- + 2e- (for charge of -2) = 32e- grand total
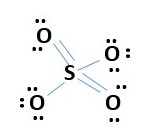
This accounts for all 32 e- = [8 bonding electrons + 4 x 6e- lone pairs]. Every atom has octet. However, formal charge calculation shows too many charges -1 for every oxygen and +2 sulfur. [4x-1 + 2= -2 charge on ion]. Need to minimize formal charges. Therefore, remove lone pair from two oxygens to form a bond with sulfur. This gives.
The formal charges are reduced now to only two -1 on the two single bonded oxygen atoms. The rest have zero or no formal charge. This low energy structure fits the formula SO4-2 very. well. This is fine because if we proceed further to make two more double bonds we will get,
The -2 formal charge on sulfur is less favorable because it is less electronegative than oxygen. Therefore, Oxygen should carry the negative formal charge and not sulfur. Also VESPR prediction for 4 bonds or e- pairs around Sulfur implies sp3 hybridization or tetrahedral structure that’s fits the previous above structure very well. The four sp2 oxygen S=O bonds around sulfurs, suggest a flat structure but would be unfavorable for a tetrahedral structure.
-
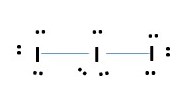
triiodide ion (I3–)
IODINE is in group 7 = 7 valence electrons
Total number of electrons needed for I3– formation.
I= 3 x 7e- = 21e- + -1e (for charge) = 22 e- grand total
Every atom has octet but we have only used 20e- [8 lone pairs + 2 bonding pairs]. we need to more electrons. IODINE at the center can have expanded octet therefore takes the extra two electrons. This gives.
VSEPR prediction for 5e- pairs or bonds around central atom implies sp3d hybridization of central atom. This gives a trigonal bipyramidal electronic structure geometry shown below.
The molecular geometric structure is the simple shape we see of the atoms and bonds without the lone pairs in the electronic geometric structure. This is a LINEAR structure for triiodide ion as shown; ignore the lone pairs.
Electron deficiency [less than octet]
6. Beryllium hydride (BeH2)
Be is in group 2 and therefore has 2 valence e-
H has one valence electron
Total electrons needed to form BeH2 is
Be = 2e-
H=2 x 1e-
Total = 4e-
VESPR prediction for 2 e-pairs or bonds around central atom is sp hybridization which is LINEAR as shown. The hydrogens are duplets (have 2 electrons) but Be has less than 8e- octet. This is fine since Be is stable with less than 8 electrons.
-
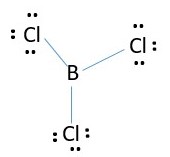
Boron trichloride BCl3
Boron is in group 3 = 3 valence electrons
Chlorine is in group 7= 7 valence electrons
Total electrons needed for BCl3 formation.
B = 3e-
Cl= 3 x 7e- = 21 e-
Total = 24e-
Boron is central atom;
Total electrons in the structure = 9 lone pairs + 3 bonding pairs = 18 + 6 = 24 e-. The chlorine atoms have octet, but Boron has only 6 electrons around it in the compound. This is fine because Boron is satisfied with less than 8 electrons.
VESPR prediction for central atom = 3 bonds or 3 electron pairs is sp2 hybridization which is flat as shown above.
Write the Lewis structure and predict the hybridization and geometry or shape [Electronic and Molecular geometry] for the following listed below and calculate the formal charge on each atom.
OZONE (O3), Sulfur trioxide (SO3) , Azide ion (N3–) and Ammonium ion (NH4+)
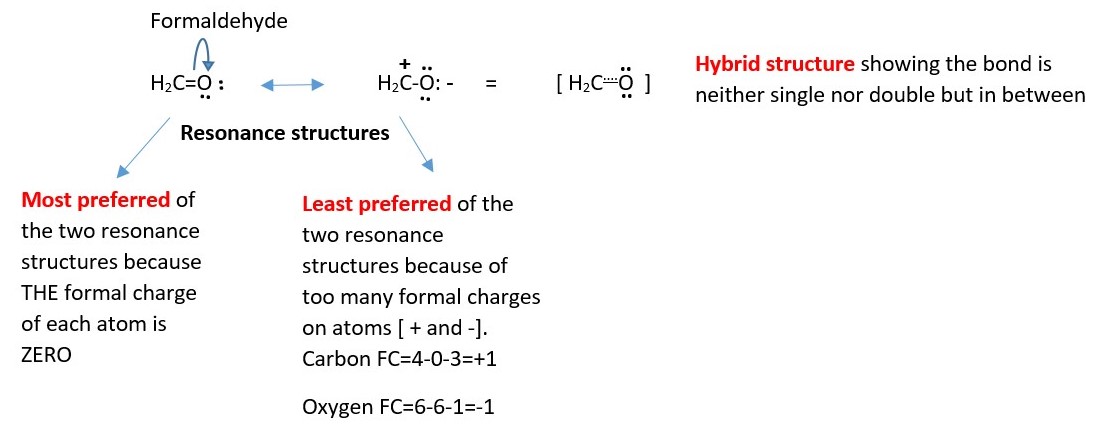
RESONANCE STRUCTURES
These are different structures of the same compound that is written by moving electron pairs on two bonded atoms from lone pair to bonded pair and vice versa in the structure.
RULES
-
Structure with least number of formal charge is most preferred
-
Most electronegative atom in the compound is preferred to carry NEGATIVE charges
-
Most electropositive atom should carry POSITVE formal charge
-
SUM of the formal charge must be equal to the charge on the ion or ZERO for a molecule or compound.
-
Do not break any permanent bond between two atoms and form a new bond with a different atom in the compound.
Accurate representation is a hybrid structure that is neither of the resonance structures.
EXAMPLES OF RESONANCE
AZIDE ION
Resonance structures of Azide ion