MOLE CALCULATIONS : MASS , MOLES , MOLAR MASS
Mass of 1 Carbon-12 atom= 12 amu
12g Carbon-12 isotope = 1 mole CARBON-12 atoms
6.023 x 1023 particles = 1 mole
6.023 x 1023 CARBON-12 atoms = 1 mole CARBON-12 Atoms
Mass of 1 mole = Molar mass [on periodic table]
22.4L = 6.023 x 1023 particles = Molar volume
Mole = amount of substance compared to 1/12th mass of one atom of CARBON-12 isotope.
MOLES
Mass of element on periodic table.
Mass of element compared to 12g of Carbon-12.
Carbon-12 scale:
12g of Carbon-12 isotope/12 = 1.0g
SIMPLE EXPERIMENT FOR CALCULATING MOLAR MASS OF ELEMENTS:
Use the box container shown below .
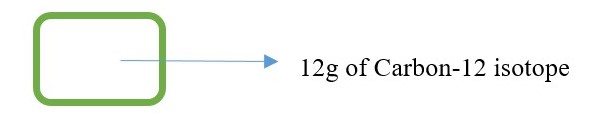
The container should be very rigid and should not allow any expansion.
Fill the container with finely powdered carbon. Ensure stiff packing with no air spaces.
Mark the level when the mass of the carbon powder in the container is 12.00g
This carbon will contain all the carbon isotopes -12 , C-13 and C-14. [Best result if you have only 12g of carbon-12 isotope].
Find Molar mass of every element:
Clean and dry container.
Fill container with any element up to the level previously marked for the 12.00g of carbon and weigh it.
The mass of element = total mass – mass of container = Mass of 1.0 mole of the element. = MOLAR MASS of element. [approximately]
This simple experiment should be very easy with powdered iron, or zinc or copper metals or even gold. However, for diatomic elements such as Nitrogen gas N2, or Chlorine gas Cl2, Hydrogen gas H2, Oxygen gas O2 and liquid bromine, Br2 . you have to divide the mass by 2 to get the approximate molar mass.
You can use the raw values from this weighing without the percent abundance as Approximate Average Molar Mass of the element.
Actual Molar mass of element = Value of the sum of [ mass of all isotopes of elements X percentage abundance of occurrence in nature] = mass of element on periodic table (a.k.a. MOLAR MASS or average atomic mass unit of element).
Approximate values for elements on periodic table; H=1g, Na = 23g , O=16g , N=14g , Cl=35.5g
AVOGADRO’s LAW = Mass of 1.0mole of substance contains 6.023 x 1023 particles.
MOLE FORMULA
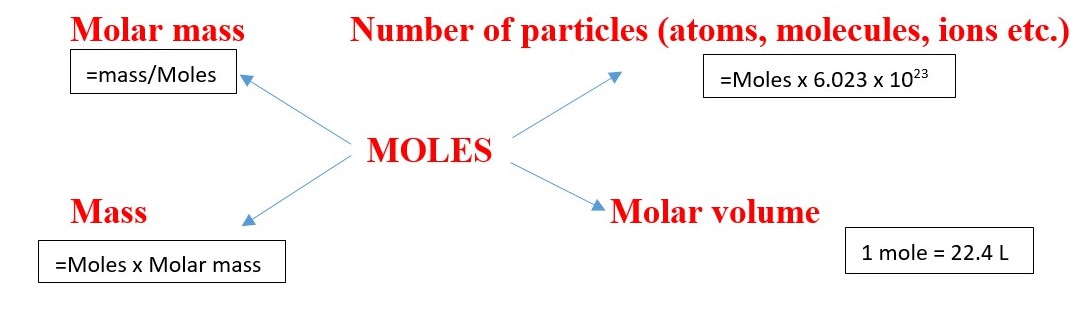
Molar mass = mass/moles
Mass = moles x molar mass
Moles= mass / Molar mass
CALCULATION OF MOLES AND MASS OF COMPOUNDS
-
Calculate the percent by mass of Sn and Oxygen in SnO2
% element = Total Mass of element in compound x 100
MASS OF COMPOUND
= Molar mass of Sn x 100
Molar mass SnO2
2. How many moles are there in 47.4g Ca
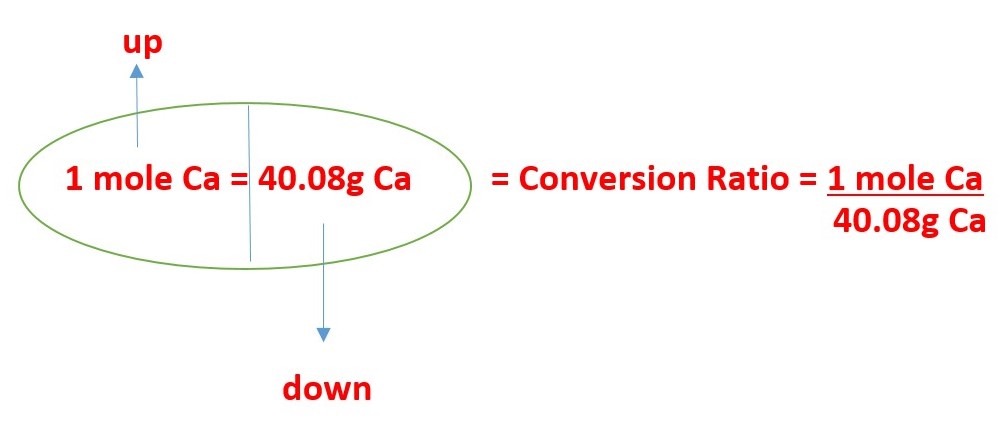
LOOK on periodic table for conversions; 1 mole Ca = 40.08g Ca,
Make a Conversion ratio = needed unit up/other unit down.
Needed unit is MOLES; therefore,
Conversion ratio = Mole ( up ) / Grams (down)
Answer
Given amount [47.4g] x Conversion ratio
=47.4g Ca x [1 mole Ca/40.0gCa]
3 . How many grams of gold are in 11.5 moles of Gold Au.
Look on periodic table ; 1 mole Au = 197g Au , needed unit is grams
Conversion ratio = grams upwards / other unit down
= 197g Au/1 mole Au
Given amount = 11.5 moles Au
Answer = given amount x CR = 11.5 mole Au x [197g Au/1 mole Au]
4.
How many atoms in 20.3 moles sulfur(S).
1 mole = 6.023 x 1023 particles, we need particle [atoms] up in Conversion ratio (CR);


