Organic reaction mechanisms
A covalent single bond is made of a SHARED pair of electrons.
This can be represented in many different forms of Lewis structures.
Example of a covalent bond representation between atom A and atom B involving sharing of a pair of electrons [:] .
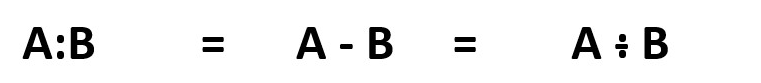
Organic molecules can be formed by breaking or formation of covalent bonds. This can occur in two different processes:
1. HETEROLYTIC process involves formation of full charges ( negative (-) and positive (+) charges.
-
HOMOLYTIC process occur when each atom takes away a single electron form free RADICALS.
Homolytic bond breaking is so simple and does not involve formation of charges. This can be represented by half arrows.
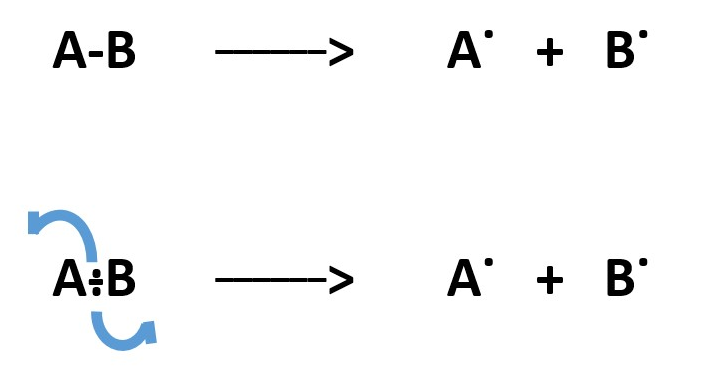
Covalent bond formation between two radicals occur when each of the two radicals donate an electron to form a shared electron pair .
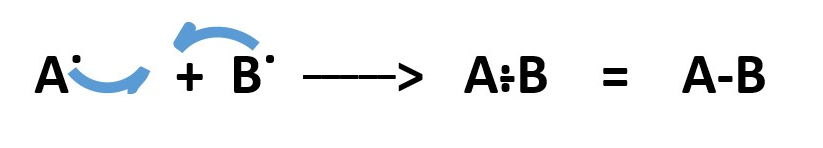
In heterolytic bond breaking, one atom takes away all the bonding electron pair and gets a lone pair of electrons as a negative charge [ .. ] . The other atom becomes deficient by two electrons acquires a positive charge [ + ] .

Heterolytic bond formation is like Lewis acid Base reaction. One atom donates a pair of electrons into an empty orbital of another atom to form a bond. The direction of the source of electron pair to the other atom is represented by a full curved arrow (shown as blue here).
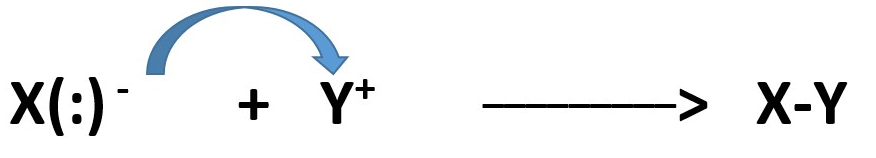
The atom with the electron pair can be called a base or a nucleophile depending on the situation. [what it does] .
If it just donates its electrons to Hydrogen atom bonded to another atom in molecule and the hydrogen loses it bonding electron pairs on the molecule and becomes attached to new atom. The hydrogen leaving as without its electrons is called a PROTON and the atom or the molecule that snatched the proton is called a BASE.
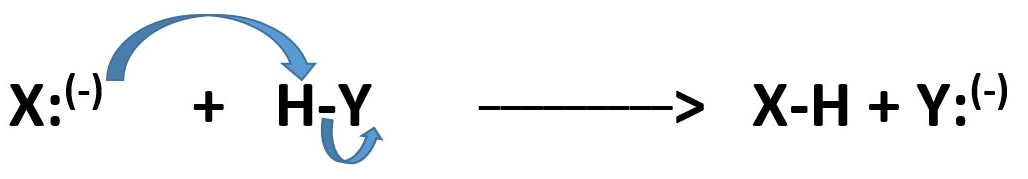
The atom or molecule or ion that donated electron pair is acting as a NUCLEOPHILE if becomes attached to the other atom or molecule or ion to form a new molecule.
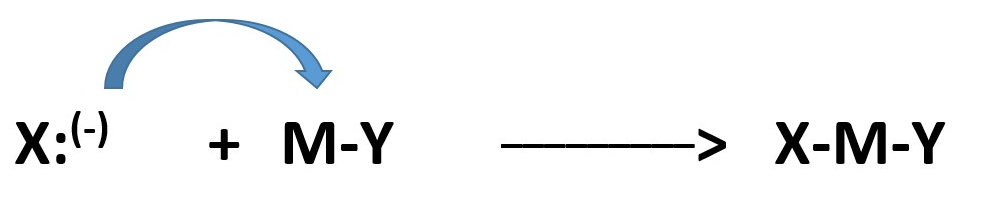
Another species (atom or group) already attached to the recipient atom (M) is expelled to satisfy the valency of the recipient atom (M).
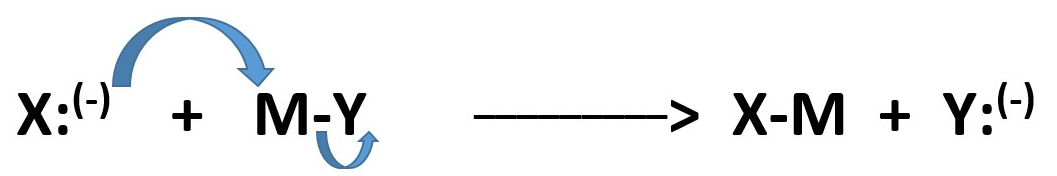
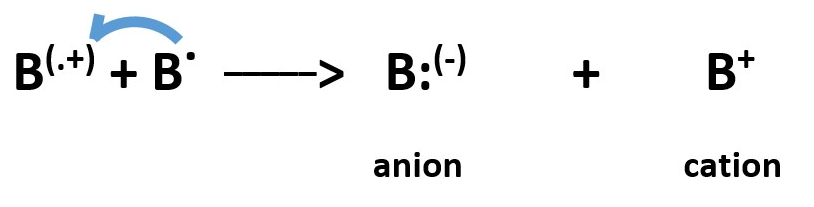
At higher levels , you can find a combination of homolytic process to produce charges . This occur by reaction between two radicals when one radical can donate an electron to another radical. The donor forms a full positive charge but the recipient gets a full negative charge.
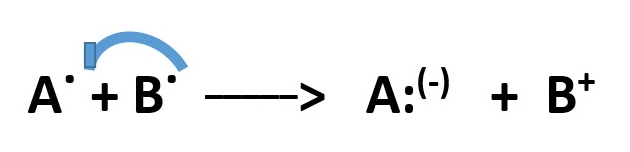
Further reactions can occur when a radical donates an electron to a species that already has a full negative charge to form a RADICAL ANION and the radical becomes a cation.
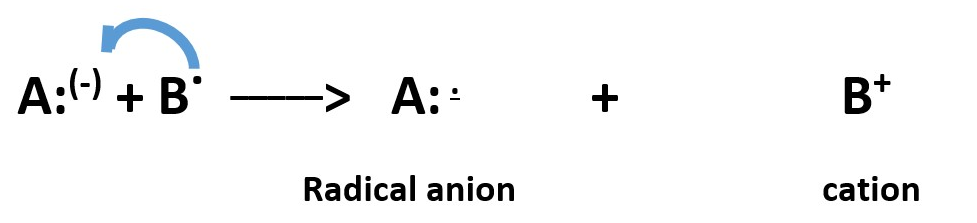
A radical can donate an electron to a species that has a full positive charge to form a RADICAL CATION and the radical becomes a cation.
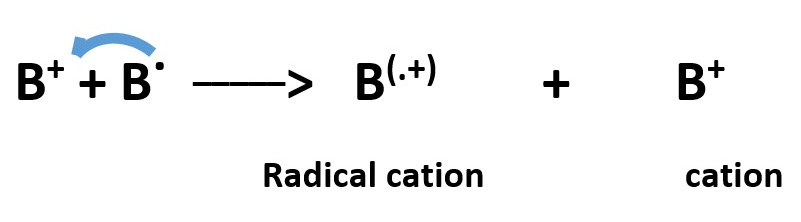
A radical cation can accept an electron from a radical to become an anion and the radical becomes a cation.
In organic reaction mechanisms involving heterolytic bond cleavage and bond formation , the products are formed by substitution or elimination process .
Substitution or elimination depends on good base or good nucleophile used. chemical species that are good bases can accommodate the electrons very strongly but share it poorly. Good or strong nucleophiles share their electrons very well. Therefore, for the reaction to proceed, the conditions should favor the incoming species, in terms of being a good base or nucleophile than leaving group. In a typical example, a Hydroxyl group (OH) is a stronger base and stronger nucleophile than Cl- or Br- or water. Therefore, –OH should substitute Cl– in a reaction and not the other way round.
Thiol ( SH ) is a weaker base but a stronger nucleophile than –OH, because Sulfur can share its electrons better than oxygen. Alkyl amines are better nucleophiles and stronger bases than alcohols, since Nitrogen can share its electrons more readily than oxygen.
The types of substitution and elimination reaction mechanisms are listed below.
SN1 substitution.
-
Formation of cation from substrate [rate limiting step]
-
Promoted by PROTIC or ionic or polar aprotic solvents since the ionic and H-bonding interactions stabilize substrate cation intermediate. This provides some solvation energy to help bond dissociation energy required in rate limiting step. [breaking away of leaving group in substrate]
-
Good leaving groups
-
Stable carbocation formation [3o>2o>1o>CH3+] [highly branched substrate are more favorable]
-
Reaction rate depends on concentration of only the substrate.
-
Promoted by less bulky , weak base, weak nucleophiles. e.g. water , alcohols , Ammonia etc.
-
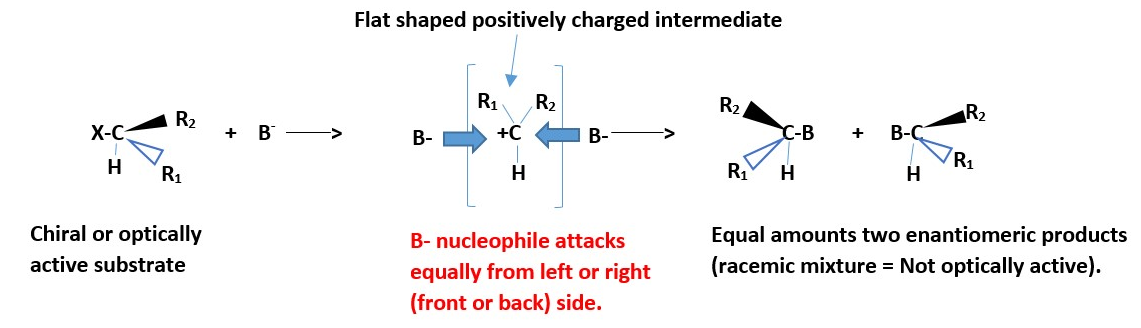
Not stereospecific nor stereo-selective. If substrate is optically active, the product will be a racemic mixture: equal amount of stereo-isomeric products because the flat or planar positive charged ion intermediate will be attacked equally on both left or right sides (front and back) by the nucleophile.
-
Promoted by higher temperatures . [This adds some energy to help bond dissociation energy required in rate limiting step. [breaking away of leaving group in substrate].
X-CHR3 + B– ______> X– + [ +CHR3 ] + B– _______> B-CHR3
[Most important / slowest = Rate determining step]
B- = Nucleophile
EXAMPLE:
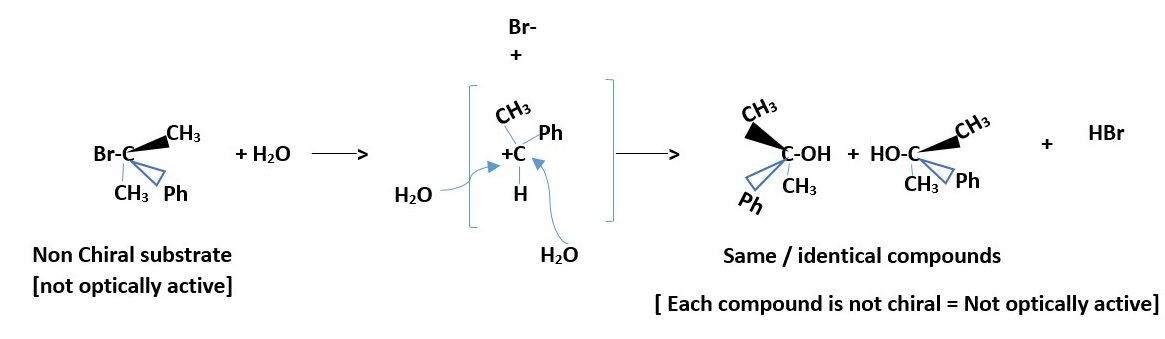
Ph-C(CH3)2Br + H2O/acetone / heat ___________> Ph-C(CH3)2OH + HBr
RACEMIZATION occur for optically active starting compound (substrate).

TRY EXAMPLE :
Write mechanism for reaction below :
Tert-butyl bromide + methanol/acetone ___________> tert-butoxy methane
60oC
SN2 substitution.
-
Occur in whole single concerted step
-
No cation formation by substrate
-
Good leaving groups
-
Preferred substrate [3o< 2o< 1o< CH3+] [less branching substrates; more preferred]
-
Promoted by PROTIC or Aprotic non-polar solvents.
-
Reaction rate depends on concentration of two species. BIMOLECULAR [Substrate and nucleophile]
-
Stereospecific; stereochemistry of starting compound is reversed in the product. [Product has opposite stereochemistry to that in starting substrate material]
-
less bulky, Weak base; strong nucleophile. e.g. less hindered amines (e.g. Alkyl amines)
-
Promoted by higher temperatures.
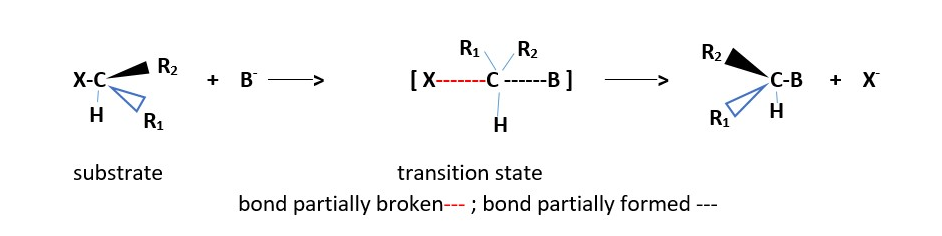
EXAMPLE :
Reaction of (S)-2-bromo-2-phenylbutane and sodium hydroxide
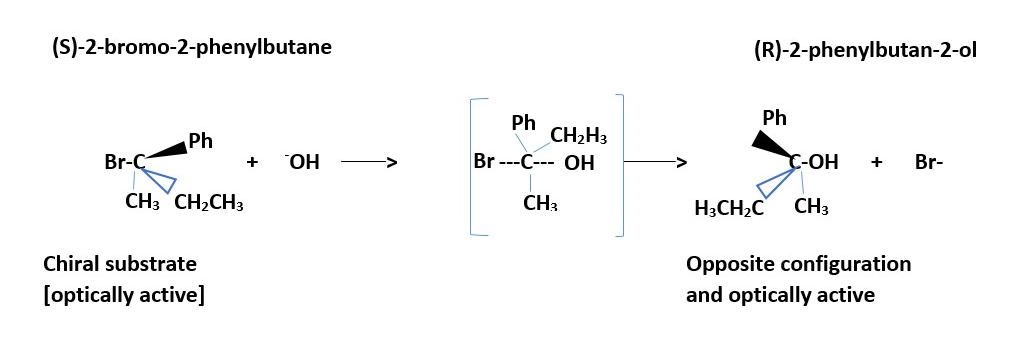
Try and write the mechanism of the reaction below.
( R )1-bromo-1-methyl butane + sodium methoxide /DMSO/60oC _________>
( S )1-bromo-1-methylbutoxymethane
Elimination (E1)
-
Formation of cation from substrate [rate limiting step].
-
Reaction rate depends on concentration of only the substrate.
-
Bulky, Weak base , weak nucleophile. e.g. water ,alcohols , NH3, Br- , Cl- , Pyridine etc.
-
Promoted by lower temperatures.
-
Stable carbocation formation [3o>2o>1o>CH3+] [highly branched substrate are more favorable]
-
Promoted by PROTIC or ionic or polar aprotic solvents; because ionic and H-bonding interactions stabilize substrate cation intermediate. This provides some solvation energy to help bond dissociation energy required in rate limiting step. [breaking away of leaving group in substrate]
-
Good leaving groups in SYN or anti conformation to the H removed .
-
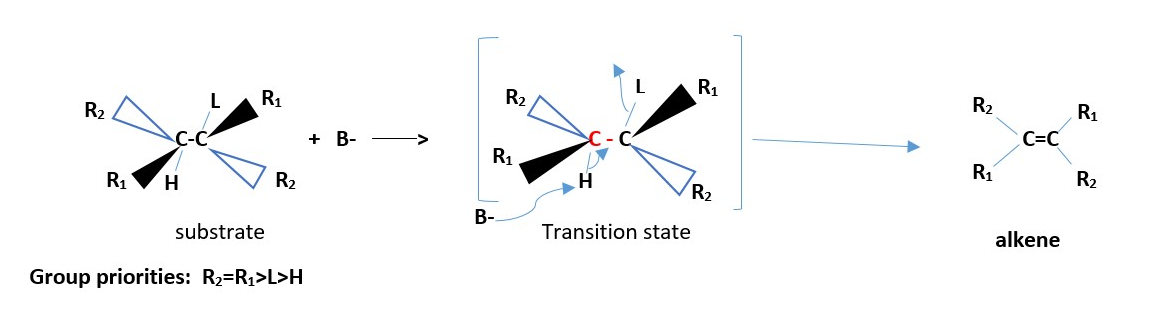
For optically active substrates, the leaving group L can be SYN or anti to H ; carbocation formed in substrate has free rotation. Cis and trans alkene products form.
GENERAL MECHANISM:
NON-CHIRAL SUBSTRATE / NOT OPTICALLY ACTIVE
Only one alkene is formed ; [neither cis nor trans]
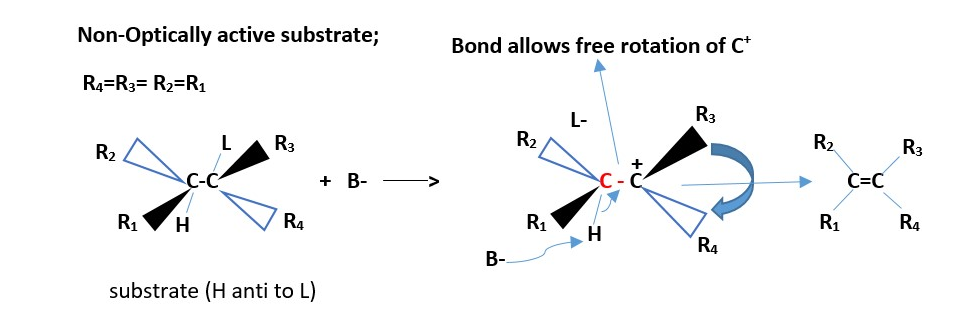
EXAMPLE:
H2SO4 + CH3(OH)(CH3)C-CH(CH3)CH3
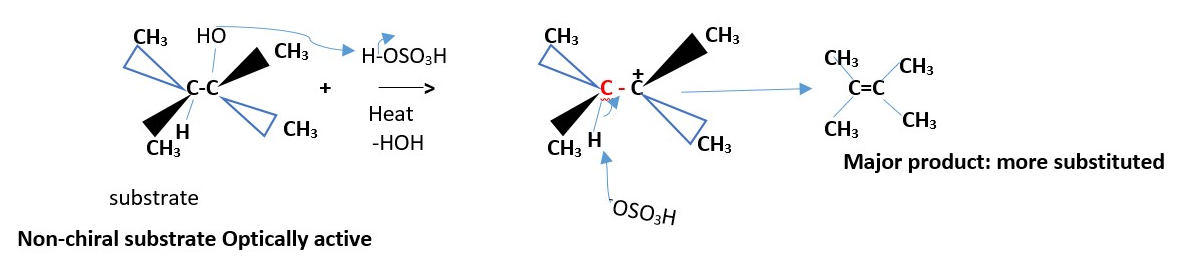
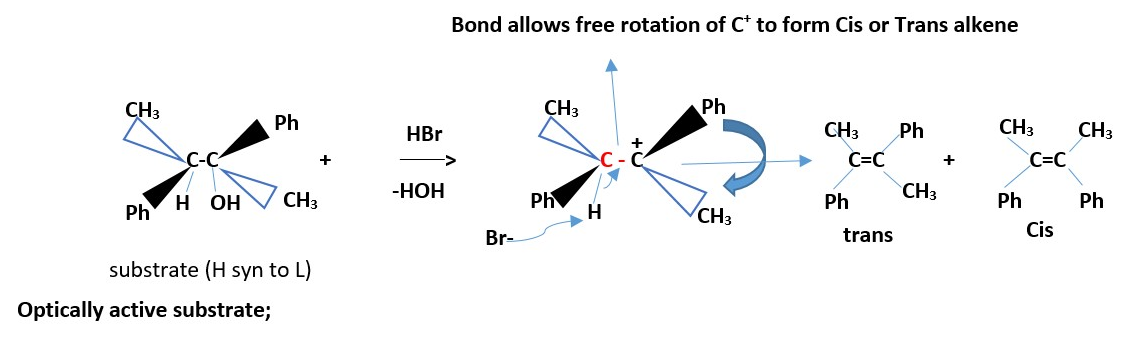
H can also be syn to leaving group OH to get the same results.
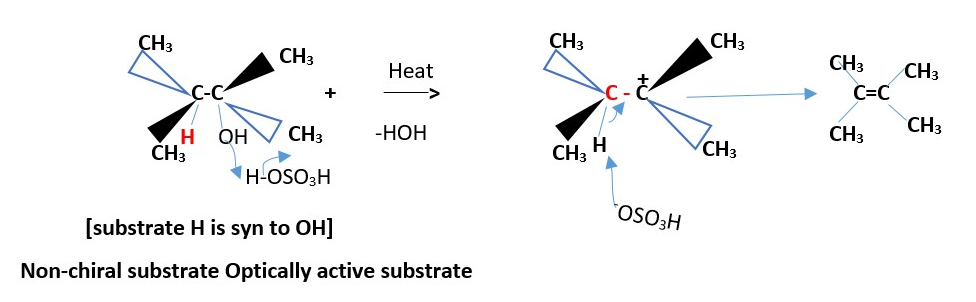
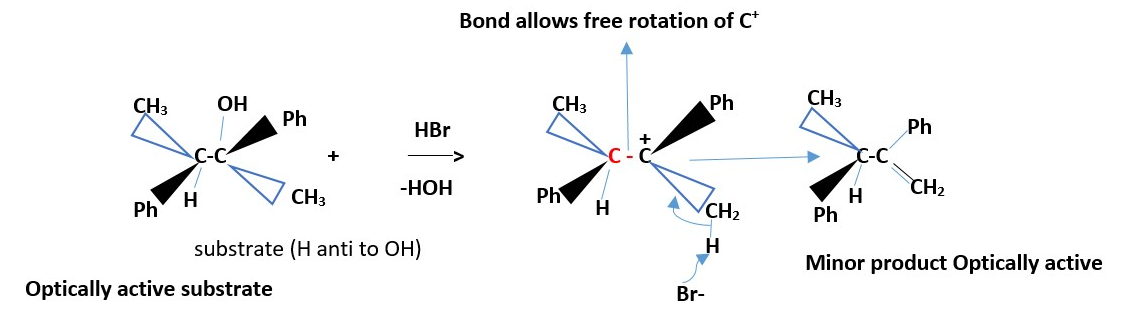
Formation of minor product [least substituted alkene]
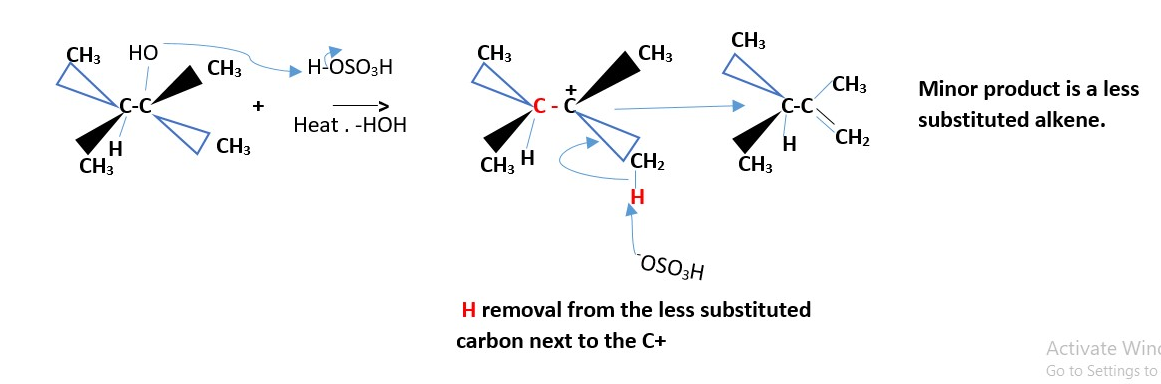
Optically active substrate
If two groups are the same on one carbon (that is if only one of the carbons involved in the reaction is chiral, then the results give the same alkene.
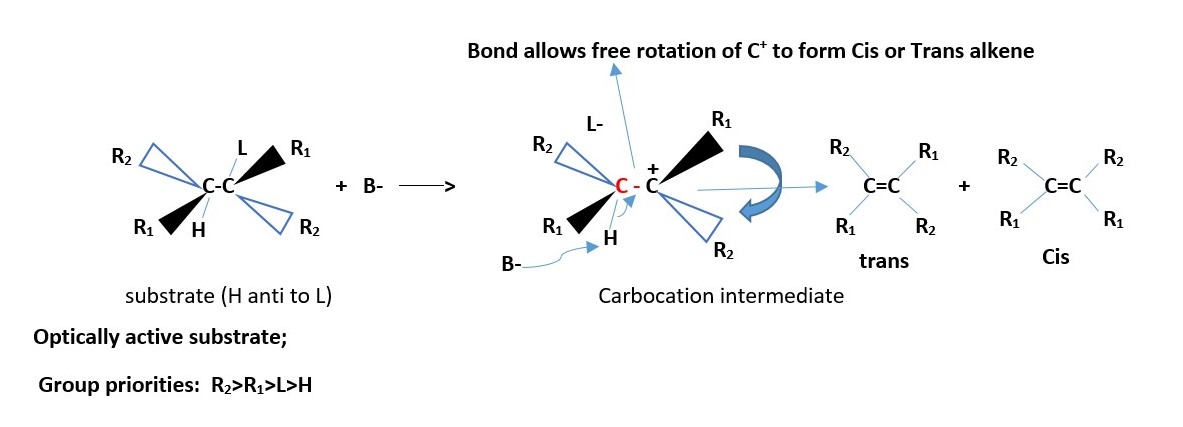
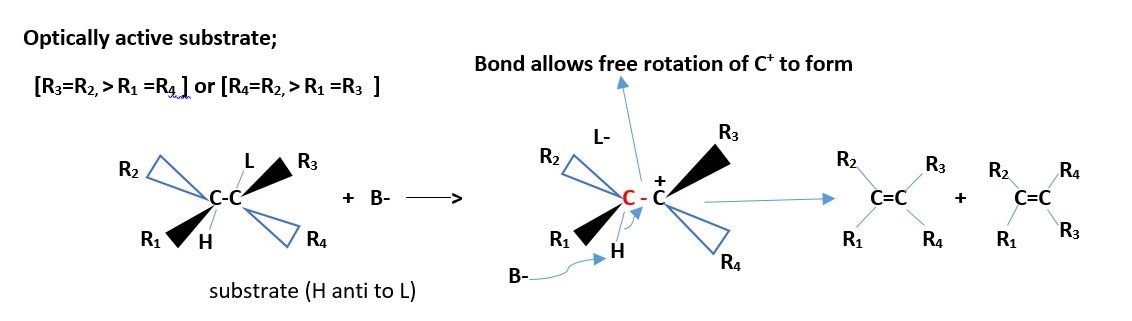
If two different groups attached to each carbon involved the reaction. We have two chiral carbons, then the results are Cis and Trans EZ –isomers of alkenes. However, if all the four groups are different on the two carbons involved in the reaction then we can only have EZ-alkene isomers.
The other ways is the SYN conformation that also gives the same products.
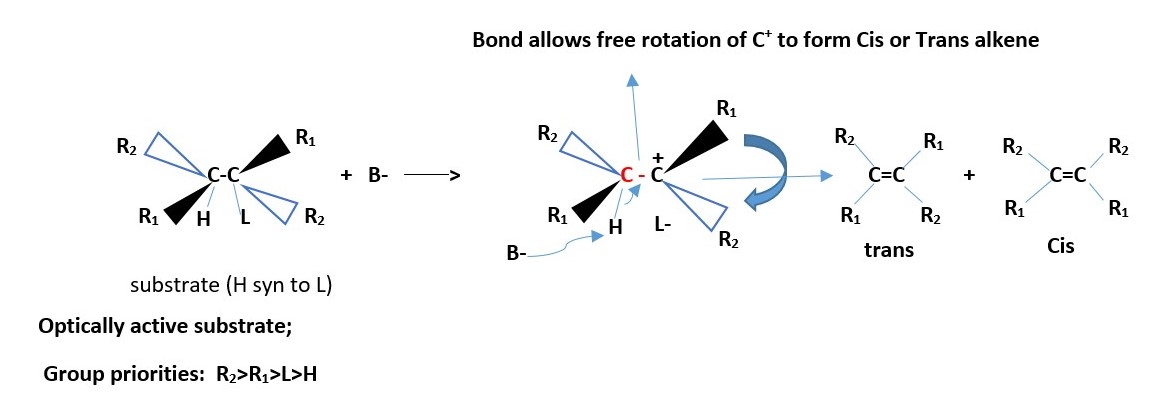
The diastereomer of the above compound will react to give the same results due to free rotation allowed for the C-C+ bond above.
[ Try and draw the mechanism ] .
Example:
HBr + CH3(Ph)HC-C(OH)(Ph)CH3 _________> CIS + TRANS ALKENE
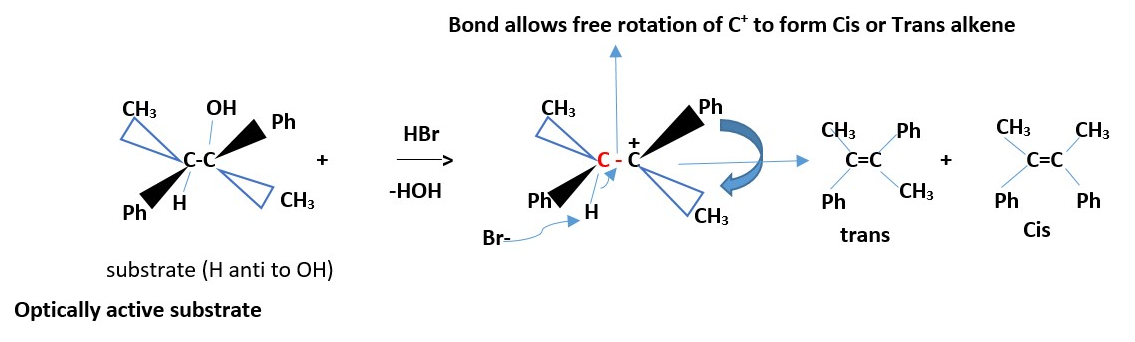
The minor product will be optically active and no cis or trans alkene forms.
Mechanism:
Elimination (E2)
-
Occur in one whole single concerted step.
-
No cation formation by substrate.
-
Promoted by highly branched substrate [3o >2o > 1o >CH3+] .
-
Reaction rate depends on concentration of two species. BIMOLECULAR [Substrate and nucleophile]
-
Bulky, strong base; weak nucleophile. e.g. -OH , –OCH3, NH2– , LDA , etc.
-
Promoted by by PROTIC or aprotic non-polar solvents.
-
Good leaving groups. Due to steric effects, leaving groups ( H and L) must be anti-conformation to each other. This leaves two parallel p-orbitals that can form the pi-bond.
-
Stereospecific; If substrate is optically active, H is always anti to Leaving group L ; only TRANS ALKENE products are formed..
-
Promoted by lower temperatures.
NON-CHIRAL NON OPTICALLY ACTIVE SUBSTRATE
EXAMPLES :
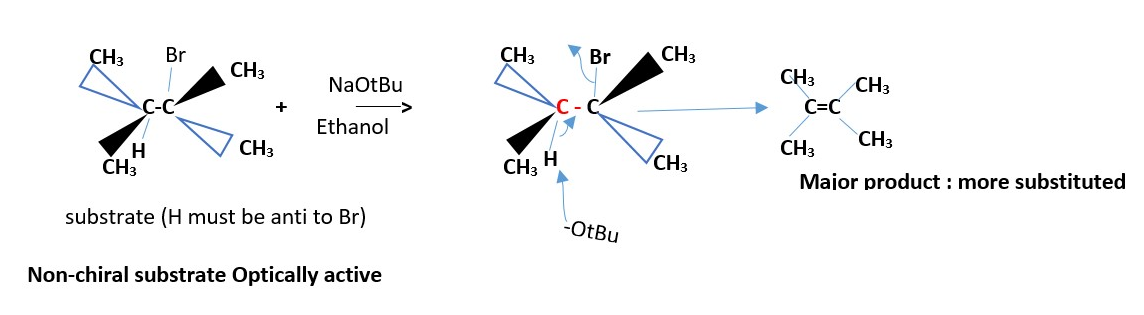
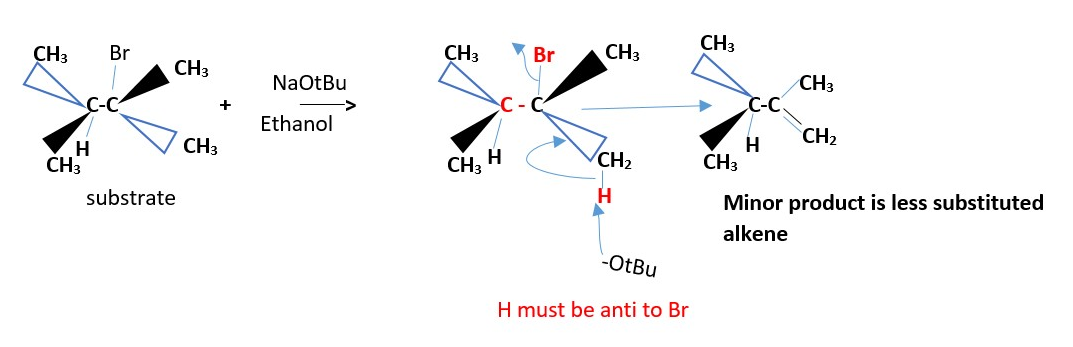
The minor product is a less substituted alkene . Neither cis nor trans.
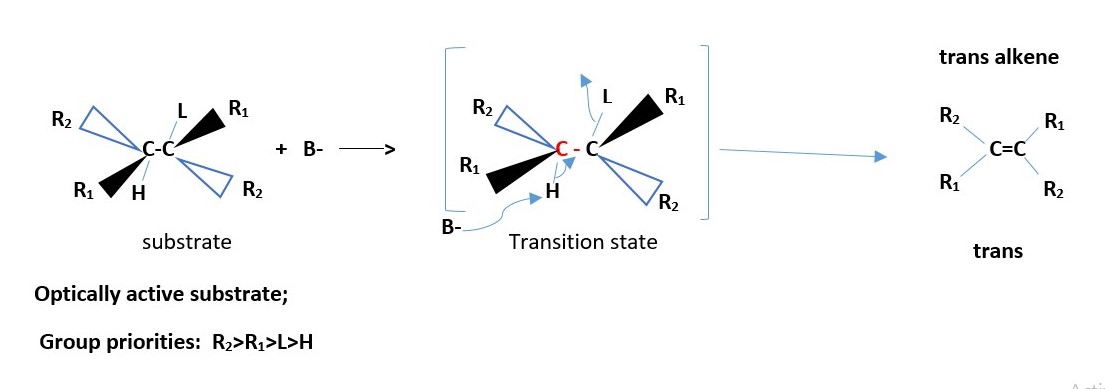
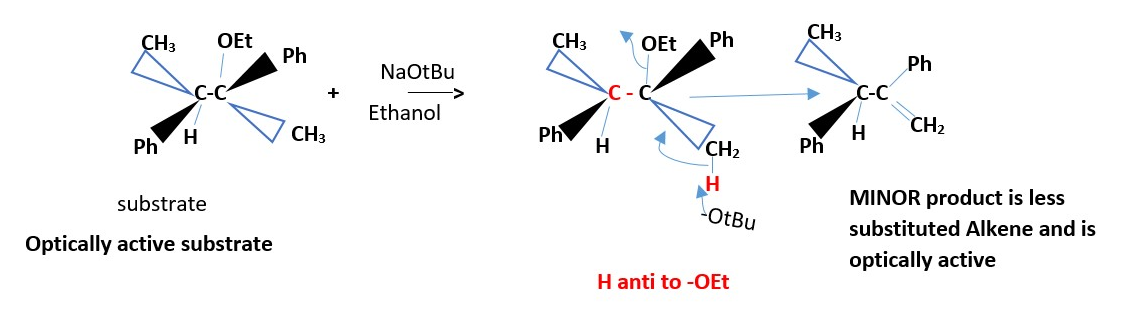
OPTICALLY ACTIVE SUBSTRATE :
The major product is a TRANS ALKENE.
[H anti to L]
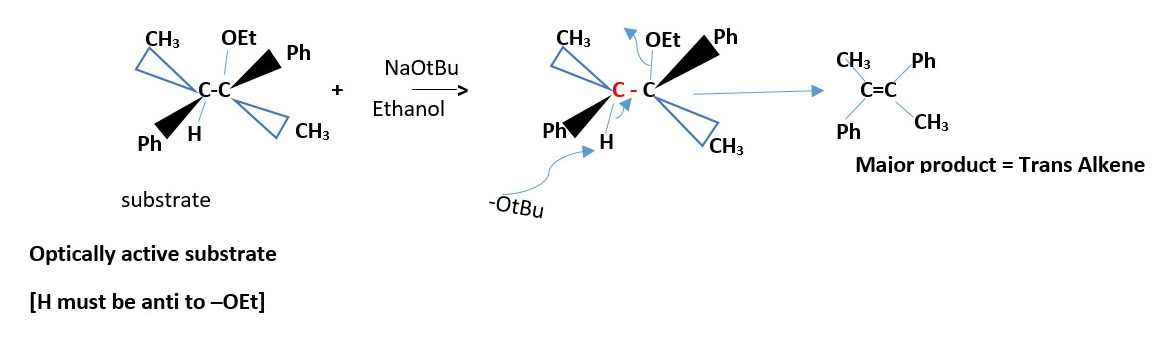
EXAMPLE :
NaOtBu / ethanol + CH3(Ph)HC-C(OEt)(Ph)CH3 _________________> trans alkene
The diastereomer will react to give a CIS alkene as MAJOR product . [ Draw the mechanism ] . Repeat the mechanism by using the ENANTIOMER of the substrate and state if you get the same major product.
Draw the mechanism for formation of the minor product. Indicate if it can rotate plane polarized light (optically active).
SUBSTITUTION VS ELIMINATION :
GENERALIZED EFFECTS OF STERICS AND BASE STRENGTH
Small substrate + small WEAK base WEAK nucleophile = substitution[SN2]> elimination
Small substrate + bulky WEAK base STRONG nucleophile = substitution [SN2] > elimination
Small substrate + bulky STRONG base WEAK nucleophile = substitution<elimination[E2]
Small substrate + bulky STRONG base STRONG nucleophile = substitution[SN2] > elimination
Bulky substrate + small WEAK base WEAK nucleophile = elimination < substitution[SN1]
Bulky substrate + small STRONG base WEAK nucleophile = elimination [E1]> substitution
Bulky substrate + small WEAK base STRONG nucleophile = elimination < substitution [SN1 or SN2]
Bulky substrate + small STRONG base STRONG nucleophile = elimination [E2] > substitution
Bulky substrate + bulky STRONG base WEAK nucleophile = elimination [E2] > substitution
Bulky substrate + bulky STRONG base STRONG nucleophile = elimination [E2] > substitution
Bulky substrate + bulky WEAK base WEAK nucleophile = elimination [E1] > substitution
Bulky substrate + bulky WEAK base STRONG nucleophile = elimination < substitution[SN1]
Other factors that also contribute to competition between substitution and elimination includes the solvent used , the temperature of reaction etc.
SN1-cb ( SN1-carbanion )
These involve formation of carbanions in the substrate instead of cations as shown in the above reactions.
EXAMPLES
A strong base will abstract or remove an acidic H (proton) on a carbon alpha to a carbon attached to an electronegative atom [ oxygen or nitrogen etc.].
e.g. H-C(R)2-( R)C=O. This leaves a lone pair electron as a negative charge on the alpha carbon –(:)C-C=O. The chemical species (atom or ion) containing the negatively charge alpha carbon is sometimes called a CARBANION. [ –(:)C-C=O is simply written as –C-C=O ]
Carbanions are usually useful nucleophile for inter or intra-molecular reactions in organic synthesis.
CH3ONa + CH3-C(=O)CH2CH3 _______> –CH2-C(=O)CH2CH3 + CH3OH
There are two alpha carbons attached to the carbon bearing the oxygen [C=O] The primary carbon in red on the left was chosen because its more acidic and easier to access than the secondary carbon on the right.
CH3ONa + CH3-C(=O)CH2C=O(O)CH3 ________> CH3-C(=O)- –CHC=O(O)CH3
The middle alpha carbon is more acidic than the alpha carbon on the left because it is attached to two carbons bearing electronegative atoms [C=O].
Carbanion is also stabilized by resonance with the oxygen. More resonance structures that can be written implies more stability and thus ability of the carbon to form a carbanion.
Carbanion reactions
The reactions are similar to substitution reactions mentioned above.
CH3ONa + CH3-C(=O)CH2CH3 _______> –CH2-C(=O)CH2CH3 + CH3Br _____> CH3CH2-C(=O)CH2CH3 + NaBr
Kinetics versus thermodynamics
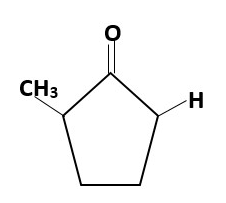
Kinetics refer to how fast by which we can form a product first. Thermodynamics refers to formation of the most stable major product. Sometimes the kinetic product is same as thermodynamic product but sometimes due to steric hindrance they are not the same.
kinetic enolate
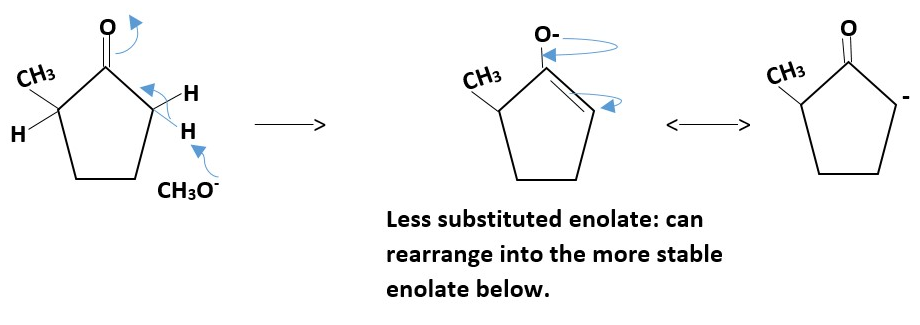
Thermodynamic enolate
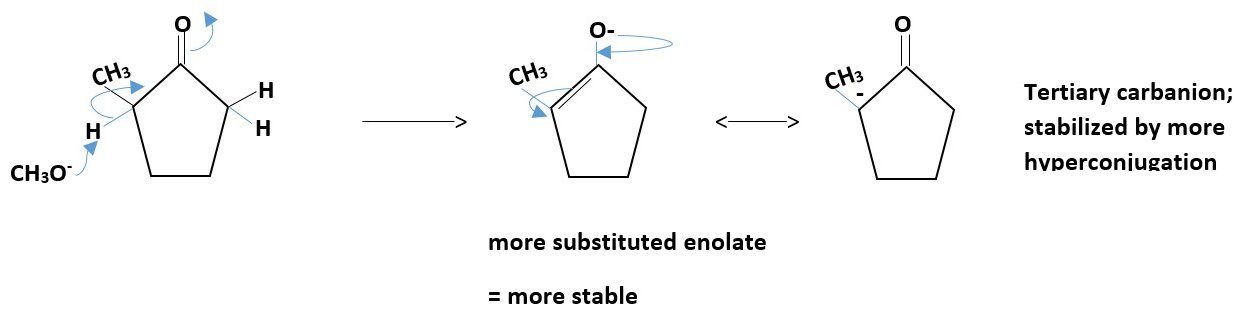
Sometimes the chemical environment used can lead to formation of either kinetic or thermodynamic major intermediate or product.
A huge bulky base such as tert-butyl lithium or LDA will react with the substrate to form mostly kinetic enolate due to steric issues but a smaller size bases such as sodium hydroxide will form the thermodynamic enolate an equilibrium mixture of kinetic and thermodynamic enolates equal amounts.
A Weak base can form more thermodynamic enolate when coupled with other favorable conditions. E.g. Methyl amine , NaSCH3 , Pyridine , DBU etc.
Chemical structures can be modified to form predominantly the thermodynamic enolate as major product. E.g.
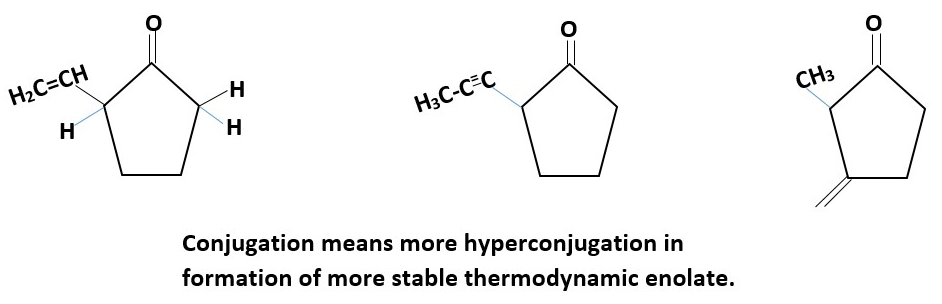
CH3-C(=O)C(H)2C=C-CH3 CH3-C(=O)CH2CH=CH2
Drawing the stable conformation of the cyclopentane ring shows some of the sterics involved. Draw the Enveloped and twisted conformations.
Show that, the 1,3-diaxial interactions between the hydrogens increases in kinetic enolate than thermodynamic enolate.
ACIDITY
Some compounds have hydrogens near on carbons near electron withdrawing groups. The hydrogens are easily removed as protons by a base a chemical reactions.
Examples:
CH3-C(=O)CH2C(=O)CH3 , CH3-C(=O)CH2C(=O)OCH3
The alpha hydrogens in the middle labelled red are more acidic than the terminal hydrogens and likely to be removed to form the thermodynamic enolate.
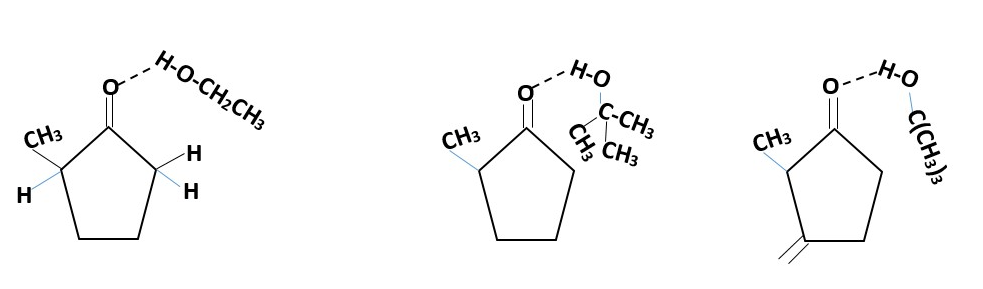
SOLVENTS:
Protic solvents creates H-bonding with the carbonyl such that the solvent molecule hinders formation of kinetic enolate. The methyl group sterically pushes more H-bonding to occur at the right side of the molecule.
Example use of ethanol or tert-butanol as solvent
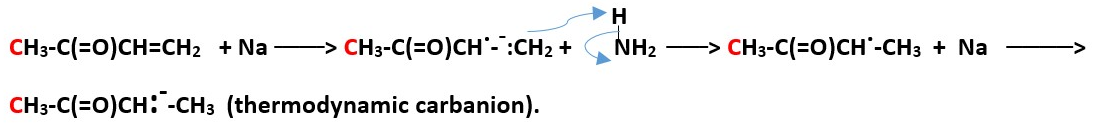
Free radicals or single electron donors e.g. Li or Na reaction with a,b- unsaturated ketones
Sodium and Lithium have loosely held single valence electron that easily donate to other atoms
e.g. CH3-C(=O)CH=CH2 + Na/NH3 .
There are other special reagents that are made to be used for synthesis of thermodynamic enolates.
Other carbanions include formation of Grignard reagents, Alkyllithiums, Lithium dialkyl cuprates etc
Grignard:
Alkyl halide reaction with magnesium = Grignard reagent
RBr + Mg ________> RMgBr = R: – -MgBr
e.g. CH3CH2-Br + Mg _______> H3C(H2)C:– -Mg-Br
Alkyllithiums
e.g. CH3CH2Li [Ethyl lithium].
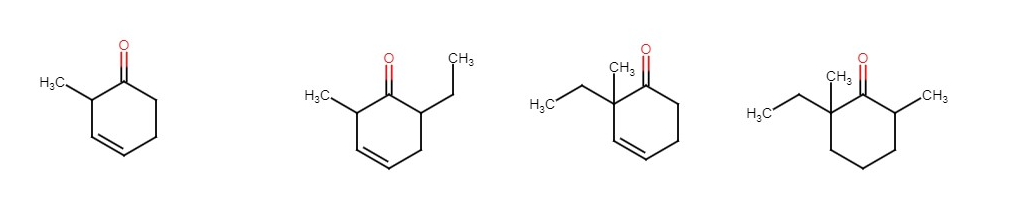
Try using the compound A below to make compounds B, C and D.
| Starting compound A | B | C | D |