ACIDS , BASES , SALTS , BUFFER SOLUTIONS, pH and pI
Salts, Acids , Bases and Buffers and pH
There are several ways to define an acid ,base or salt . One definition is Bronsted acid defines an acid as a proton donor). However, the Lewis acid definition makes an acid an electron pair acceptor. On the other hand , a base is a proton acceptor (Bronsted) or electron pair donor (Lewis definition). Salt is usually formed when an acid reacts with a base and one of the hydrogens is replaced by a metal atom. Bronsted acids contain hydrogen ions that can reacts in proton transfer reactions. Examples include of Bronsted acids include HCl, H2SO4 , HCIO4, H3PO4 .
Lewis acids do not necessarily have to contain any hydrogen ions and examples are AlCl3, FeCl3 and BF3.
Bronsted bases usually contain hydroxyl group (OH) and examples include NaOH , KOH, Mg(OH)2. However, a Lewis base will be any species with electron pair that can be donated to react to form a bond. Examples include Sodium hydride (NaH) , Ammonia (NH3) , all the alkali and alkaline earth metals and their oxo-compounds. For example, sodium metal (Na) , Sodium oxide (Na2O), Magnesium metal Mg, and Magnesium oxide (MgO) . Transition metals and their oxo-compounds can also be classified as Lewis bases.
All Bronsted bases can also function as Lewis bases vice versa. However, not all Lewis acids function as Bronsted acids. Sodium hydroxide can react with HCl to form sodium chloride (NaCl) salt and water.
A Buffer is a weak base with the salt of its conjugate acid or a weak acid with a salt of its conjugate base.
pH is a measure of hydrogen ion concentration. pH=-Log [H+]. Smaller pH value is more acidic but larger value is more basic. pH 7 is neutral.
Strong acid dissociates completely HA _________> H+ + A+
pH is –log [H+]
Strong bases dissociate completely
e.g. NaOH _________> Na+ + OH–
pOH= – log[OH–]
pH + pOH = 14
pH=14- pOH
WEAK ACIDS
Do not dissociate completely in solution. Equilibrium is reached between ions and solid salt or molecules.
HA < = > H+ + A–
k = Acid dissociation constant = Product concentration / reactants concentration = [H+][A–] /[HA]rem
At equilibrium the HA produces equal H+ and A- , [H+] = [A–]
Amount of initial acid remaining at equilibrium = [HA]rem = [HA] – [H+] ,
Sometimes, this is approximately equal to [HA] because of small dissociation.
To avoid using quadratic equation.
Therefore, K = [H+]2/[HA]rem , or sometimes just, k = [H+]2/[HA]……1
Standard values of k for weak acids are calculated and known.
From eqn 1, [H+] = (k[HA]rem)1/2
PH = – Log [H+] = -Log [(k[HA]rem)1/2] = -[½] ([ logk ]+ log[HA]rem])
PH = ½ (-logk –log[HA]rem)
=1/2 (pk- log[HA]rem)
Weak bases (B)
B + HOH < = > BH + OH-
Base dissociation constant kb = [products] / [reactants] = ([ OH–][ BH])/([ HOH ][B]rem) ; water is large excess solvent and ionization is taken as negligible compared to the base so ignore water
Kb = ([OH–][ BH])/([B])
At equilibrium, Base forms [OH-] = [BH] , [B]rem= [B]o –[OH–]
kb= ([OH–]2)/([B]rem)
([OH–]= (kb[B]rem)1/2
pOH=- log[OH–] = -log (kb[B]rem)1/2)
=1/2(-logkb – log[B]rem)
=1/2 (pkb – log[B]rem)
From ionic product of water, HOH < = > H+ + OH–
10-7 = 10-7
[H][OH-]=10-14= Kw =k x kb
pkw = pk + pkb
SALT SOLUTIONS
Salts of strong acids and bases dissociate completely in solution. The ions are hydrated and exist as neutral species and do not undergo any further interactions with water molecules to any extent as generate more protons or hydroxyl ions in solution.
NaCl _______> Na+ + Cl– ………neutral
Salts of weak acids and bases do not dissociate completely and can form acidic or basic solutions
Acid salt = Salt of strong acid and weak base = forms acidic solutions
NH4Cl ______> NH4+ + Cl –
Weak conjugate acid neutral
NH4+ + HOH < = > NH3 + H3O+
Weak base strong acid
k = [NH3][H3O+]/ [NH4+]
Basic salt = salt of strong base and weak acid = forms basic solutions
NaOOCCH2CH3 ________> Na+ + –OOCCH2CH3
Neutral weak conjugate base
HOH + –OOCCH2CH3 _________> HOOCCH2CH3 + OH–
Weak acid strong base
kb = [HOOCCH2CH3 ][ OH–] / [ –OOCCH2CH3 ]
BUFFER SOLUTIONS
Acid buffer: Weak Acid + its salt of strong base or Weak acid and its salt.
E.g. HOOCCH2CH3 + NaOOCCH2CH3
k = [H+][ NaOOCCH2CH3] / [HOOCCH2CH3]
[H+] = k[HOOCCH2CH3] / [NaOOCCH2CH3]
Basic buffer: Weak base + its salt of strong acid or Weak base and its salt.
E.g.CH3NH2 + CH3NH3+Cl
kb = [OH][ CH3NH3+Cl] / [CH3NH2]
[–OH] = kb[CH3NH2] / [CH3NH3+Cl]
Principle of electroneutrality and quantitative analysis:
Concentration of positive and negative charges must balance out to achieve electro-neutrality in a system.
Calculate the pH of a solution of a solution containing 0.03 mol of CaF2 in 1.0 L water.
Answer:
CaF2 is sparingly or partially soluble in water.
CaF2 (aq) < = > Ca2+ (aq) + 2F– (aq) Ksp = 4.0 x 10-11 = s + 2s
where s=solubility of CaF2 = concentration of Ca2+ ions, 2s = concentration of F- ions
[Ca2+ ] [F–]2 = Ksp = 4.0 x 10-11
[s] [2s]2= Ksp = 4.0 x 10-11
4s3 = Ksp = 4.0 x 10-11
s= [(4.0 x 10-11 )/4 ]1/3 = 0.0002154435M = solubility of 1mol CaF2 in 1L solution , 0.03 mol CaF2 will be [0.03mol / 1mol ] x 0.0002154435M = 0.0000064633 M
Therefore [Ca2+ ]= 0.0000064633 M , [F–]= 2 x 0.0000064633 M = 0.0000129266 M
F- ion undergoes basic hydrolysis;
F– + H2O < = > HF + OH– Kb = ?
We know HF(aq) < = > H+ + F– Ka = 6.8 x 10-4
Therefore Kw=kbka ,
kb = Kw/ka = (1.0 x10-14 ) / (6.8 x 10-4) = 1.471 x 10-11 ….. F– + H2O < = > HF + OH–
Kb= [HF][OH–]/ [F–]
Concentrations are very low that we have to take into account self-dissociation of water
H2O < = > H+ + OH– [The OH– from water will add to the OH– from F– hydrolysis]
[X][X+10-7]/[ 0.0000129266-X] = 1.471 x 10-11 ,
We cannot approximate because of very low concentrations
[X][X+10-7] = [1.471 x 10-11 ][ 0.0000129266-X]
[X][X+10-7] = [1.471 x 10-11 ][ 0.0000129266-X]
X2+10X=1.9015×10-16-1.471 x 10-11 X
X2 +10x +1.471 x 10-11 X -1.9015×10-16 = 0
X2 +1.0015×10-7 X -1.9015×10-16 = 0
quadratic equation solution ;
X = (-1.0015×10-7 +/- [( 1.0015×10-7)2 +4(1)( 1.9015×10-16 )] ½ )/2(1)
= 1.86639 x 10-9 M
Therefore [HF]= 1.86639 x 10-9 M
[OH–] = 1.86639 x 10-9 M +10-7M=1.01866 x10-7M
[F–] = 0.0000129266 – x = 0.0000129266M -1.86639 x 10-9 M = 0.0000129247M
pOH=-log [1.01866 x10-7] = 6.9919691 pH=14 – 6.9919691 = 7.008130933, [H+]=9.816 x 10-8M
Charge balance: [H+] + 2[Ca2+] = [OH–] + [OH–] + [F–]
Mass balance: [HF] + [H+] + 2[Ca2+] = [OH–]+ [OH–] + [F–]
1.86639 x 10-9 M + 9.816 x 10-8M + 0.0000129266 M = [1.01866 x10-7M+ 0.0000129247M]
1.30266 x 10-5 M = 1.30266 x 10-5 M
This proves that mass balance is correct.
The solution is almost neutral due to low concentration of the insoluble salt CaF2 .
P.S. Try and calculate the pH of 0.03mol KF in 1.0L water and compare result with CaF2 explain the difference between the results.
Question Example:
0.0350mol KH2PO4 was added to 0.040mol KOH in 1L water.
Calculate the concentration of each species in solution and the pH of the solution.
ANSWER:
Charges or ions in the solution:
KH2PO4 Consist of K+ + H2PO4 –
KOH ________> K + + OH–
Mole ratio ; mol K+ / mol OH- = 1/1 , this implies 1mol K+ = 1mol OH-
[K+] = [OH–] for a solution of KOH only.
However, reaction between OH- ions and hydro-phosphate ion occur in solution to produce more ionization:
OH– + H2PO4 – _____> HPO4 -2 + H2O
Extra OH remain reacts further to form more ions.
OH– + HPO4 -2 _______> PO4 -3 + H2O
All OH– ions used up in this step. However, a typical complete hydrolysis reaction between water and the phosphate ions reforms some OH-ions ;
PO4 -3 and some HPO4 -2 reacts with water [reversible] = hydrolysis
HPO4 -2 + H2O < = > H3PO4– + 2OH–
Therefore, the mole ratio = mol OH–/mol HPO4 -2 = 2/1
1mol OH– = 2mol HPO4 -2 ,
this implies [OH–] = 2[ HPO4 -2 ]
PO4 -3 + H2O < = > H3PO4 + 3OH–
mol OH–/ mol PO4 -3 = 3/1
1mol OH– = 3mol PO4 -3
this implies [ OH–] = 3 [ PO4 -3 ]
Charge balance: list any possible ion present in solution including dissociation of water:
[H+] + [K + ] = [OH– ] + [H2PO4 – ] + 2[HPO4 -2 ]+ 3[ PO4 -3 ]. This is just listing all possible ions involved.
Mass balance:
This involves quantitative amounts of any species that contribute significantly to the concentration of the solution.
[K+] = [OH–] + [H2PO4 – ] + 2[HPO4 -2] + 3[PO4 -3]
Concentration or sum of the concentration of the positive charges should be equal to concentration or sum concentration of negative charges.
Calculations should prove this:
KOH is a strong electrolyte and dissociates completely to ions. Therefore, 0.04 mol KOH Contains 0.04mol K+ ions and 0.03mol OH– ions.
KH2PO4 is a strong electrolyte and dissociate completely . Therefore, 0.035mol KH2PO4 contains 0.035mol K+ ions and 0.035mol H2PO4–.
The OH- (0.03mol) base picks or reacts with protons in H2PO4– (0.035mol) to form HPO4-2 , (0.035mol) and H2O. Excess OH- (0.005mol) that remains converts some amount of HPO4-2 ions to PO4-3 ions
(0.005mol), leaving [0.035-0.005] mol or 0.030mol HPO4-2 ions.
After the complete dissociation, we have to calculate the amounts of each ion formed from each stage of the reversible incomplete basic hydrolysis reactions with water shown.
H2PO4– + H2O < = > H3PO4 + OH- …….…… kb =1.3 x 10-12
0.0M [all reacted with OH–] 0.0M
Hydro phosphate ion hydrolysis
HPO4-2 + H2O < = > H2PO4– + OH- ………………. kb =1.6 x 10-7
[0.030-X] M X X (where X is equilibrium amounts formed)
kb =1.6 x 10-7 = [H2PO4–][OH-]/[ HPO4-2]
1.6 x 10-7 = [X][X]/[ 0.030-X]
The value of kb is very small, therefore approximate 0.030-X = 0.030M
Therefore, 1.6 x 10-7 = [X][X]/[ 0.030] , X = 6.928 x 10-5M
Therefore, this step forms
[H2PO4–]=[OH-]= 6.928 x 10-5M
[ HPO4-2] = [0.020-X] =[0.030-6.928 x 10-5 ]M = 0.029930718M
Phosphate ion hydrolysis
PO4-3 + H2O < = > HPO4-2 + OH- . …… kb = 2.1 x 10-2
0.005-X 0.029930718+ X X
kb = 2.1 x 10-2 = [HPO4-2][OH- ]/[ PO4-3 ]
1.0 x 10-2 = [0.029930718+X][X ]/[ 0.005-X ]
Value of kb is huge so we cannot approximate so we have to work through;
X(0.029930718+X) = 0.021(0.005-X)
X2-0.050930718X-0.000105= 0
Use quadratic formula solution:
X = (-0.050930718 +/- [(0.050930718)2 + 4(0.000105)]0.5 ) / 2(1)
X = 0.001984313M
[HPO4-2]= 0.029930718+ X = [0.029930718+0.001984313]M= 0.031915031M
[OH- ]= 0.001984313M
[ PO4-3 ] = [ 0.005-X ] = [ 0.005- 0.001984313]M=0.003015687M
Total Sum of ions listed;
All ions are contained in 1.0L volume so every mole of substance forms a concentration of Molarity M units.
[K+] = 0.04M + 0.035M = 0.075M
[OH– ]= 0.001984313M + 6.928 x 10-5M = 0.002054M
[H2PO4–]= 6.928 x 10-5M
[HPO4-2]= 0.031915031M
[ PO4-3 ]= 0.003015687M
Check mass balance to see if our calculation is correct electro-neutrality of charges.
[K+] = [OH–] + [H2PO4 – ] + 2[HPO4 -2] + 3[PO4 -3]
0.75M = (0.002054M) + (6.928 x 10-5M ) + (2 x 0.031915031M) + (3 x 0.003015687M)
This proves we are correct.
pOH =-log[OH–] = -log (0.002054) = 2.69
pH = 14-2.69 = 11.31
[H+] = 10-11.31 = 4.9 x 10-12 M
AMINO ACIDS: pH AND pI
Living organisms contain proteins
Proteins consist of Amino acids: Amine group and Carboxylic acid group
AKA alpha-amino acid because amine group is on alpha carbon next to carboxylic group.
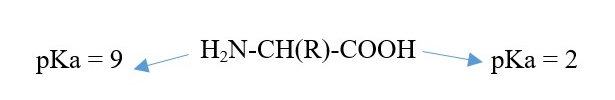
Ionization occur in solution of pH different from pKa of amino or acid group. The R group can affect the pKa if it is an amino or a carboxylic acid.
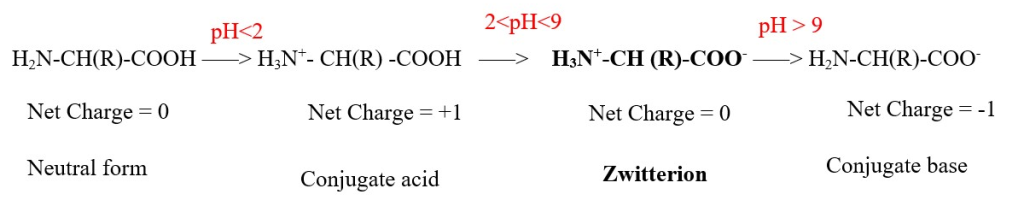
Zwitterion = equal number of positive and negative charges = total net charge = 0. This is Isoelectric form or Iso-ionic form. This is used to calculate the pI or isoelectric pH.
Isoelectric pH = pI = average mean value of pKa values of amino and acid groups excluding pKa of the main alpha COO- group of ZWITTERION.
Titration curve of a typical amino acid (AA) sample with different amounts of base.
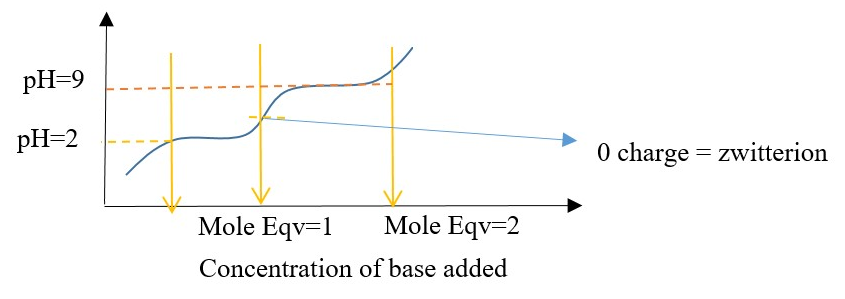
ELECTROPHORESIS:
Using electricity to separate amino acid or proteins.
pH< pI = positive form of AA= [will migrate to negative electrode when electricity is applied]
pH > pI = negative form of AA= [will migrate to positive electrode when electricity is applied]
pH = pI = net 0 charge =neutral or zwitterion form of AA= [ will not move when electricity is applied]
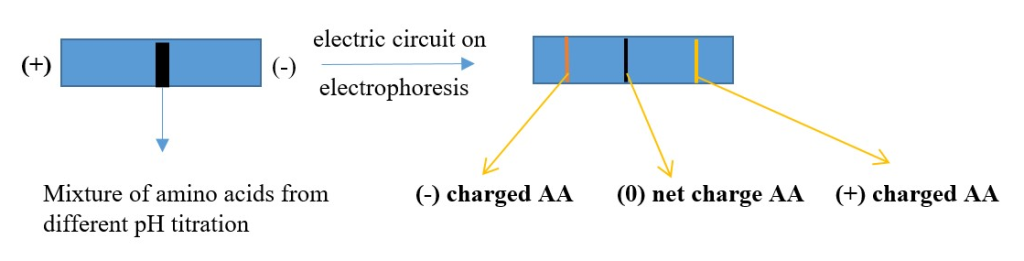
Several methods of electrophoresis exist
Example is Isoelectric focusing electrophoresis: charged proteins moving through electric field in a pH gradient support till pI of AA is reached then AA stops moving. Different proteins consist of different AA thus we get different travel distance of protein AA.
Question example :
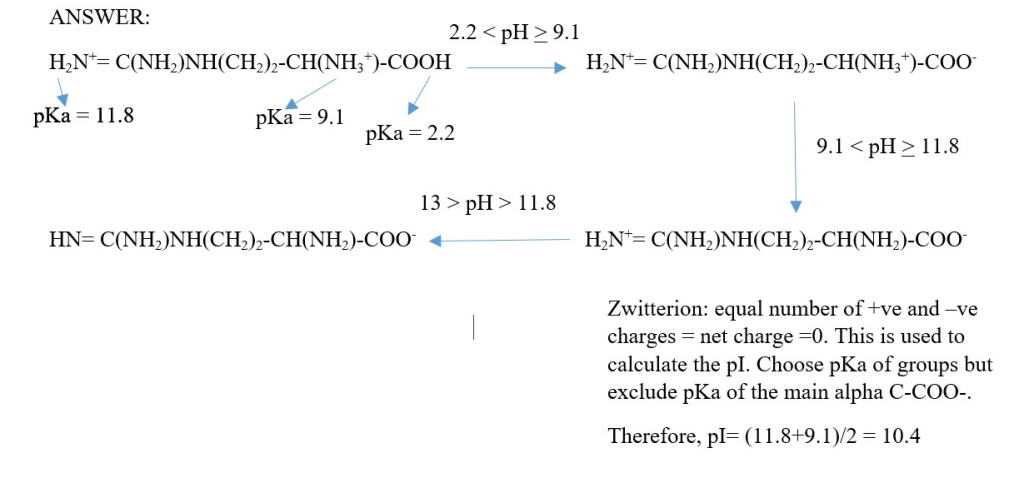
Calculate the pI of arginine and show the different forms of the amino acid at different pH values.
Answer;